Submit Documents via the CTP Portal Next Generation
Note: On Jan. 17, 2025, FDA transitioned from the CTP Portal website to the CTP Portal Next Generation website. At this time, the CTP Portal Next Generation facilitates the upload and transmission of eSubmitter-generated submissions for all submission types. In the future, users will be able to utilize new web forms within the CTP Portal Next Generation for Premarket Tobacco Product Application (PMTA) and Substantial Equivalence (SE) reports, while the FDA's eSubmitter tool will continue to support all other submission types. FDA will provide additional information when available.
On this page:
- What Is CTP Portal Next Generation?
- What Can I Do in the CTP Portal Next Generation?
- Managing CTP Portal Next Generation User Accounts
- How Do I Create a New User Account in the CTP Portal Next Generation?
- How Do I Obtain a CTP Portal Next Generation Account or Submit Help Desk Inquiries?
- What Responsibilities Does My Organization Have in Managing User Accounts for CTP Portal Next Generation?
- When Setting Privileges in the CTP Portal Next Generation, How Do I Know Which Specific Privileges Each User Has?
- Does CTP Portal Next Generation Have Non-IAM Privileges?
- FDA's eSubmitter Software and the CTP Portal Next Generation
What Is CTP Portal Next Generation?
CTP Portal Next Generation is a web-based platform designed for regulated entities in the tobacco industry—such as manufacturers, importers, and distributors—to electronically send tobacco product submissions to the FDA. Initially, all existing submission types will require the use of eSubmitter to package submissions, which can then be uploaded through the CTP Portal Next Generation. In the future, users will have the option to utilize newly introduced web forms within the CTP Portal Next Generation for PMTA and SE submissions, while the eSubmitter tool will remain available to support all other submission types.
Once available, the PMTA and SE web forms will offer a more integrated, efficient, and secure way to submit documents, with additional web forms for other submission types being introduced in phases.
The CTP Portal Next Generation also allows users to view detailed information about their submissions. To access the portal, organizations must designate an Industry Account Manager (IAM), who will manage and secure access to the system.
CTP Portal Next Generation
What Can I Do in the CTP Portal Next Generation?
The CTP Portal Next Generation is intended to be used by regulated tobacco industry manufacturers, importers, distributors, TPMF owners, universities, and other sponsors who send tobacco product submissions to CTP.
The CTP Portal Next Generation can be used to:
- Upload documents to CTP electronically and securely, seven days a week, with confirmation of receipt, if uploaded successfully, including:
- Ingredient Listing
- Reporting of Harmful and Potentially Harmful Constituents (HPHCs)
- Health Documents (not adverse experience or product problem reports)*
- Apply to Market
- Respond to CTP inquiries
- View a list of your submissions and FDA regulatory letters, including:
- Date CTP received your submission
- Assigned FDA tracking number for your submission
* For information regarding section 904(a)(4) requirements, please refer to the Final Guidance: Health Document Submission Requirements for Tobacco Products.
A tutorial video which provides an overview of the CTP Portal Next Generation and how to use it, including how to find your application’s submission tracking number (STN) online will be available soon.
The CTP Portal Next Generation cannot be used for the following:
- Register tobacco product manufacturing establishment(s) and list your product(s)
- The CTP Portal Next Generation does not replace the Tobacco Registration and Product Listing Next Gen (TRLM Next Generation) system. Refer to the TRLM Next Generation Instructions for guidance on how to submit tobacco registration and product listing information. Note: Users must first create a TRLM Next Generation account before submitting tobacco registration and product listings.
- Gain access to the FDA Electronic Submissions Gateway (ESG)
- Gain access to the Safety Reporting Portal (SRP) for submitting Adverse Experience (AE) and Product Problem Reports
Managing CTP Portal Next Generation User Accounts
The organization is responsible for designating the Industry Account Manager (IAM), and the designated IAM is responsible for managing the CTP Portal Next Generation user accounts. While the IAM is the delegate authority, ultimately, the organization is responsible for the behavior of their employees and agents on the CTP Portal.
How Do I Create a New User Account in the CTP Portal Next Generation?
Only the organization’s IAM(s) can create a new user account.
How Do I Obtain a CTP Portal Next Generation Account or Submit Help Desk Inquiries?
Before requesting a CTP Portal Next Generation account, inquire within your organization whether an IAM has been designated. If your organization already has an IAM, contact them to request a user account. Otherwise, request an Industry Account Manager (IAM) for your organization.
For additional inquiries about CTP Portal Next Generation accounts, contact the FDA eSub Help Desk by email at CTPeSub@fda.hhs.gov and include "CTP Portal Next Generation" in the subject line or call 1-877-CTP-1373, extension 4.
When contacting the FDA eSub Help Desk to submit a ticket, please include the following information if applicable:
- Name
- Company
- Phone number
- Username
- STN (tracking number)
- A summary of the issue including what IT application is being used, URL of the site where the issue was encountered, the web browser being used, and whether the computer is a Windows or Mac.
- For Industry Account Manager (IAM) applicants, please include a tracking number and date of application.
What Responsibilities Does My Organization Have in Managing User Accounts for CTP Portal Next Generation?
Your organization is responsible for creating users and managing the list of users who have access to your organization’s CTP Portal Next Generation account and managing the permissions of each user. If an employee, attorney, or agent are no longer affiliated with your organization, the IAMs for your organization are responsible for deactivating that person’s CTP Portal Next Generation user account to prevent further access. Alternatively, if the sole IAM is departing your organization, it is the responsibility of your organization and the departing IAM to reassign the IAM role to another individual prior to their departure. This will ensure continuity of access and permissions for your organization.
When Setting Privileges in the CTP Portal Next Generation, How Do I Know Which Specific Privileges Each User Has?
CTP Portal Next Generation has three different privileges that can be given to each CTP Portal Next Generation user by the CTP Portal Next Generation Admin. The different privileges and their permissions and abilities within CTP Portal Next Generation are described in the table below:
| CTP Portal Next Generation "Privilege" | Associated Permission or Ability in CTP Portal Next Generation |
|---|---|
| Submissions –View Only | This default privilege allows users to access the Submissions section from the global navigation menu, including all sub-sections, and view the draft, sent, and published submission-related information for their organization. |
| Submissions – Create/Edit/Submit/View | This privilege provides users with the ability to submit data to CTP through the CTP Portal Next Generation. This privileges to use the Upload functionality for eSubmitter-packaged ZIP files for submission types other than Premarket Tobacco Product Applications (PMTA) and Substantial Equivalence (SE), and the ability to create, edit, and submit PMTA and SE submissions using dedicated web forms provided within the portal. |
| Administration – User Administration | This privilege allows users to create and manage user accounts for their organization. This privilege is granted to the Industry Account Manager (IAM) for their organization, and the IAM may grant this privilege to other user accounts in their organization as needed. |
FDA's eSubmitter Software and the CTP Portal Next Generation
How Do I Package a Submission Using eSubmitter?
You need to use FDA's eSubmitter software:
- Save your text documents as .pdf with optical character recognition and your data files in a data file format such as .xlsx or .xpt. FDA forms in spreadsheet format should be saved as .xlsx. Save all of your submission files to one folder location on your computer.
- Download and install eSubmitter, if you have not already done so.
Note: eSubmitter is a free tool that helps you create an electronic submission, which you can upload into the CTP Portal Next Generation. - Open eSubmitter.
- Click "create new submission." Select the eSubmitter template for the submission type you want to create:
- CTP Tobacco Product Ingredient Listing
- CTP Reporting of Harmful and Potentially Harmful Constituents (HPHCs)
- CTP Tobacco Product Health Documents
- CTP Transmittal Form for All Other Submission Types
- Follow the guided process within eSubmitter. Answer the questions and fill out the screens. You will be prompted to attach the PDF documents and data files you saved in step 1.
- At the end of the process, eSubmitter packages your submission as a compressed ZIP file. Save the eSubmitter ZIP package to your computer.
How Do I Upload eSubmitter Packages to the CTP Portal Next Generation?
- Log in to the CTP Portal Next Generation (https://ctpportal-ng.fda.gov/portal/) using your credentials.
- Navigate to the dashboard and click on "Create New Submission" to open the Create New Submission page.
- Select the "eSubmitter Upload | Submission Package" radio button.
- Click Next to proceed.
- Use the Browse button to locate the ZIP file you wish to upload. Please note:
- The CTP Portal Next Generation only accepts eSubmitter ZIP files for upload.
- Only one ZIP file can be uploaded at a time.
- Enter a description for your submission in the provided field.
Once uploaded, a confirmation message will appear in the CTP Portal Next Generation, indicating a successful upload.
Important Note:
If you do not receive a confirmation message or encounter an error, the FDA has not received your submission package. In such cases, you will need to resubmit the file.
Refer to the image below for guidance on uploading your submission package.
* The CTP Portal Next Generation provides more functionality than the ESG, and CTP recommends using the CTP Portal Next Generation. However, if you already have an ESG WebTrader account, you can still use it to submit documents to CTP.
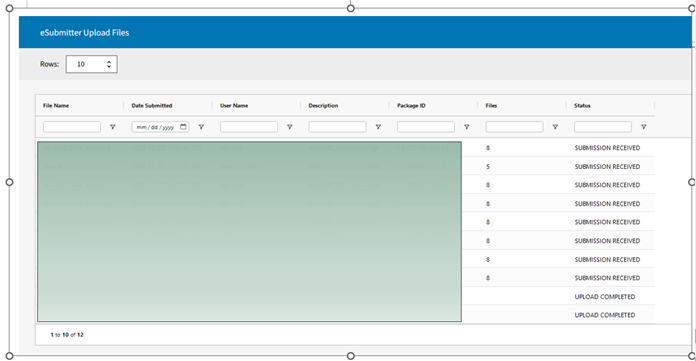
Where Can I View Information on Prior Uploads?
To view previous uploads, navigate to the Submissions tab, select Sent, and check under the eSubmitter Upload Files section (see image below).
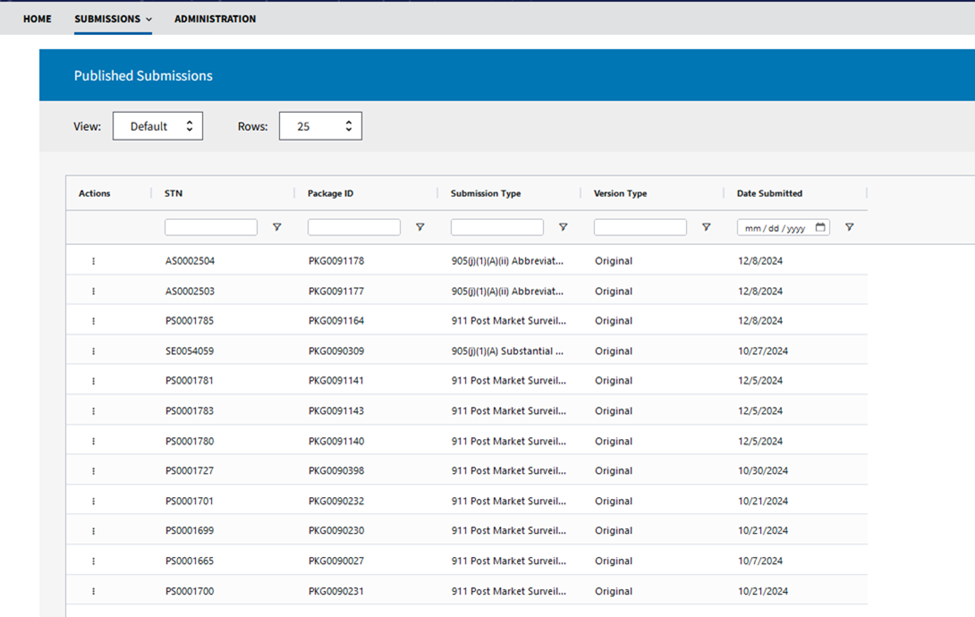
Where Can I View Information on Published Submissions?
To view published submissions, navigate to the Submissions tab, select Published (see image below).
A tutorial video on navigating the CTP Portal Next Generation will be available soon.
What Are Some Possible Causes of Being Unable to View My Organization's Uploads?
There are several possible reasons for being unable to view your organization’s uploads, including:
- The name of the organization used to set up the IAM account must be an EXACT match to the organization name submitted in the application.
- There were multiple IAM accounts created for your organization by mistake (e.g., accounts created using slightly different spelling). In this case, please contact the CTP eSub Help Desk by email at CTPeSub@fda.hhs.gov (include "CTP Portal Next Generation" in the subject line) or call 1-877-CTP-1373, extension 4.
- Your access was removed by the IAM and you no longer have access to view these uploads.
- Your account was deactivated. User account passwords need to be reset every 90 days to stay active (per FDA security protocols). If you did not reset your password in this timeframe, please contact the CTP eSub Help Desk by email at CTPeSub@fda.hhs.gov (include "CTP Portal Next Generation" in the subject line) or call 1-877-CTP-1373, extension 4.
- Your account was administratively closed by an Industry Account Manager (IAM). In this case, please contact the IAM Request Team by email at ctp-iam-request@fda.hhs.gov for further information.