Drug Trials Snapshot: CORLANOR
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the CORLANOR Prescribing Information for complete information.
CORLANOR (ivabradine)
(core' lan ore)
Amgen Inc.
Approval date: April 15, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CORLANOR is a drug used to reduce the risk of a patient going to the hospital because his/her heart is not pumping as well as it should be (also known as worsening heart failure).
How is this drug used?
CORLANOR should be used in patients who have symptoms of heart failure that are stable, a normal heartbeat with a resting heart rate of at least 70 beats per minute, and are taking a type of drug called a beta blocker at the highest dose they can tolerate.
CORLANOR is a tablet that is taken by mouth twice a day.
CORLANOR is for certain people who have long-lasting (chronic) heart failure caused by the lower-left part of their heart not contracting well.
What are the benefits of this drug?
CORLANOR reduces the risk of being hospitalized for worsening heart failure.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of CORLANOR is summarized in the table below.
Table 2. Incidence of the Primary Composite Endpoint and its Components in the Trial
|
Endpoint |
CORLANOR N=3241 |
Placebo N=3264 |
Hazard Ratio | [95% CI] | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | [% per year] | n | % | [% per year] | ||||
|
Primary composite endpoint: |
793 | 24.5 | 14.5 | 937 | 28.7 | 17.7 | 0.82 | [0.75,0.90] | <> |
|
Hospitalization for worsening heart failure |
505 | 15.6 | 9.2 | 660 | 20.2 | 12.5 | |||
|
Cardiovascular death as first event |
288 | 8.9 | 2.8 | 277 | 8.5 | 4.7 | |||
|
Subjects with events at any time |
|||||||||
|
Hospitalization for worsening heart failureb |
514 | 15.9 | 9.4 | 672 | 20.6 | 12.7 | 0.74 |
[0.66,0.83] |
|
|
Cardiovascular death as first Eventb |
449 | 13.9 | 7.5 | 491 | 15 | 8.3 | 0.91 |
[0.80,1.03] |
|
a Subjects who died on the same calendar day as their first hospitalization for worsening heart failure are counted under cardiovascular death.
b Analyses of the components of the primary composite endpoint were not prospectively planned to be adjusted for multiplicity.
N: number of patients at risk; n: number of patients having experienced the endpoint; %: incidence rate = (n/N) x 100; % PY: percent of patients who had a hospitalization for worsening heart failure or cardiovascular death per year of exposure; CI: confidence interval
The hazard ratio between treatment groups (CORLANOR /placebo) was estimated based on an adjusted Cox proportional hazards model with beta-blocker intake at randomization (yes/no) as a covariate; p-value: Wald test
Source: Adapted from CORLANOR Prescribing Information, Table 3
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: CORLANOR appeared to be similarly effective in men and women.
- Race: CORLANOR appeared to be similarly effective in whites and Asians. Because of the limited number of Black patients in the trial, differences in response for Blacks could not be determined.
- Age: CORLANOR appeared to be similarly effective in patients across age groups studied.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
The table below summarizes the subgroup analysis of the primary endpoint for the trial.
Table 3. Subgroup Analysis of Primary Endpoint*
| Subgroup | CORLANOR N=3241 |
Control N=3264 |
Hazard Ratio | 95% CI (LL, UL) |
||
|---|---|---|---|---|---|---|
| n (%)* [% per year] | Total, n | n (%)* [% per year] | Total, n | |||
| Sex | ||||||
| Male | 624 (25.3) [15.1] | 2462 | 725 (28.9) [17.8] | 2508 | 0.84 | (0.76, 0.94) |
| Female | 169 (21.7) [12.6] | 779 | 212 (28.0) [17.3] | 756 | 0.74 | (0.60, 0.91) |
| Race | ||||||
| White | 722 (25.1) [14.4] | 2879 | 835 (28.9) [17.2] | 2892 | 0.84 | (0.76, 0.93) |
| Black or African American |
9 (28.1) [19.8] | 32 | 15 (34.9) [30.2] | 43 | 0.62 | (0.27, 1.45) |
| Asian | 47 (17.5) [13.6] | 268 | 68 (25.8) [21.4] | 264 | 0.64 | (0.44, 0.93) |
| Other | 15 (24.2) [17.4] | 62 | 19 (29.2) [23.2] | 65 | 0.74 | (0.37, 1.47) |
| Age group | ||||||
| >=17 | 407 (20.6) [11.8] | 1976 | 527 (25.6) [15.6] | 2055 | 0.76 | (0.67, 0.87) |
| >=65 years | 386 (30.5) [19.0] | 1265 | 410 (33.9) [21.3] | 1209 | 0.89 | (0.77, 1.02) |
| >=75 years | 125 (33.9) [22.0] | 369 | 133 (37.7) [24.8] | 353 | 0.89 | (0.70, 1.14) |
*=primary composite endpoint was first hospitalization for worsening heart failure or cardiovascular death
% per year: percent of patients who had a hospitalization for worsening heart failure or cardiovascular death per year of exposure
Source: Company’s Clinical Trial Data
What are the possible side effects?
CORLANOR causes the heart to slow, which may cause symptoms such as dizziness and feeling tired. Much less common side effects were high blood pressure, temporary visual disturbances described as flashes of light, and a rapid, irregular heart rhythm called atrial fibrillation.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes the most common side effects in the trial.
Table 4. Adverse Drug Reactions with Rates ≥1% Higher in CORLANOR than Placebo Occurring in >1% on CORLANOR in the pivotal trial
| Adverse Reaction | CORLANOR N=3260 |
Placebo N=3278 |
|---|---|---|
| Bradycardia | 10% | 2.2% |
| Hypertension, blood pressure increased | 8.9% | 7.8% |
| Atrial fibrillation | 8.3% | 6.6% |
| Phosphenes, visual brightness | 2.8% | 0.5% |
Source: Adapted from CORLANOR Package Insert, Table 2
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of overall side effects was similar in men and women.
- Race: The risk of overall side effects was similar in whites and Asians. Because of the limited number of Black patients in the trial, differences in Blacks could not be determined.
- Age: The risk of high blood pressure in patients treated with CORLANOR is higher as age increases.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes adverse events while on treatment by subgroup for the pivotal trial.
Table 5. Subgroup Analysis of Adverse Events While on Treatment (Safety Population)
| CORLANOR (N=3260) |
Placebo (N=3278) |
|||||
|---|---|---|---|---|---|---|
| n (%) | [% per year] | Total, n | n (%) | [% per year] | Total, n | |
| Any TEAEs | 2416 (74.1) | 44.5 | 3260 | 2390 (72.9) | 43.4 | 3278 |
| Sex | ||||||
| Male | 1838 (74.1) | 44.6 | 2479 | 1837 (72.9) | 43.5 | 2519 |
| Female | 578 (74) | 44.3 | 781 | 552 (72.9) | 42.8 | 759 |
| Age group | ||||||
| >=17 | 1426 (71.6) | 41.6 | 1991 | 1457 (70.6) | 41.7 | 2063 |
| >=65 years | 990 (78) | 49.5 | 1269 | 933 (76.8) | 46.3 | 1215 |
| >=75 years | 290 (78.4) | 50.2 | 370 | 273 (77.3) | 47.9 | 353 |
| Race | ||||||
| White | 2188 (75.4) | 44 | 2900 | 2155 (74.1) | 42.7 | 2907 |
| Black or African American | 25 (78.1) | 58.2 | 32 | 30 (69.8) | 57.9 | 43 |
| Asian | 156 (58.6) | 48 | 266 | 158 (60.1) | 48.6 | 263 |
| Other | 47 (75.8) | 58.5 | 62 | 47 (72.3) | 55.8 | 65 |
TEAE=adverse event while on treatment
Source: From Company’s Clinical Trial Data
The table below summarizes the most common adverse events by subgroup.
Table 6. Most Common Adverse Events by Sex, Race, and Age (Safety population)
| % of Patients in Subgroup | Bradycardia | Hypertension | Atrial fibrillation | Phosphenes | |||||
|---|---|---|---|---|---|---|---|---|---|
| C* | P* | C* | P* | C* | P* | C* | P* | ||
| Sex, % of subgroup | |||||||||
| Male | 76 | 9.6 | 2.6 | 8.3 | 7.7 | 10.5 | 9.3 | 2.6 | 0.4 |
| Female | 24 | 11.6 | 1.6 | 11.2 | 9 | 9.8 | 7.4 | 3.2 | 1.1 |
| Age Group, % of subgroup | |||||||||
| <54> | 27 | 8.5 | 1 | 7.1 | 7.5 | 5.8 | 5.8 | 3.4 | 0.8 |
| 55 to 60 years | 24 | 8.9 | 2.8 | 8.9 | 8.1 | 9.5 | 5.9 | 2.8 | 0.5 |
| 61 to 69 years | 27 | 9.1 | 2.8 | 9 | 8.3 | 11.6 | 10.4 | 3.4 | 0.4 |
| >69 years | 23 | 13.1 | 3.2 | 11.1 | 8 | 14.6 | 13.8 | 1.4 | 0.4 |
| Race, % of subgroup | |||||||||
| White | 89 | 10.3 | 2.6 | 9.7 | 8.6 | 11 | 9.3 | 2.9 | 0.6 |
| Black or African American | 1 | 9.4 | 0 | 9.4 | 4.7 | 12.5 | 7 | 0 | 0 |
| Asian | 8 | 8.6 | 0.8 | 2.6 | 2.3 | 4.1 | 4.5 | 2.2 | 0.4 |
| Other | 2 | 8.1 | 0 | 1.6 | 6.2 | 3.2 | 6.2 | 0 | 0 |
*C = CORLANOR
*P = Placebo
Source: Analysis provided by Division
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved CORLANOR based on evidence from a clinical trial of 6505 patients with long-lasting heart failure. The trial was conducted at 667 sites in 38 countries in Europe, Asia, Australia, and South America. There were no sites in the United States. The same trial was used to evaluate both the benefit of CORLANOR as well as its side effects.
Figure 1 summarizes how many men and women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Extracted from Company’s Clinical Trial Data
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Extracted from Company’s Clinical Trial Data
Table 1. Baseline Demographics by Race (Efficacy Population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 5771 | 88.7% |
| Black or African American | 75 | 1.2% |
| Asian | 532 | 8.2% |
| Other | 127 | 2.0% |
Source: Extracted from Company’s Clinical Trial Data
Figure 3 summarizes how many patients by age were enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age (Efficacy Population)
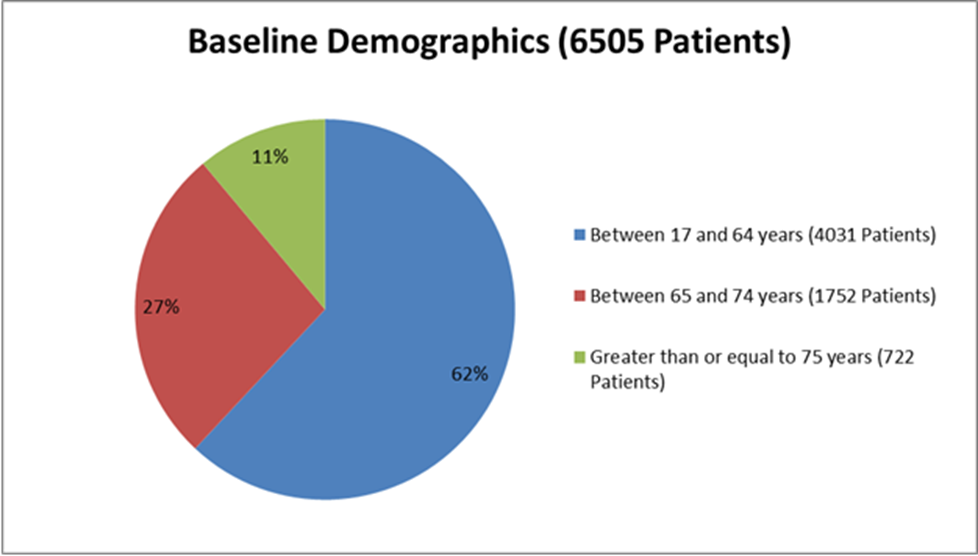
Who participated in the trials?
The table below summarizes baseline demographics for the trial. The efficacy and safety populations were similar.
Table 7. Baseline Demographics for the Pivotal Trial (Efficacy Population)
| Subgroup | Treatment Group (N=3241) |
Control (N=3264) |
Total (N=6505) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 2462 (76) | 2508 (76.8) | 4970 (76.4) |
| Female | 779 (24) | 756 (23.2) | 1535 (23.6) |
| Age | |||
| Mean years (SD) | 60.7 (11.2) | 60.1 (11.5) | 60.4 (11.4) |
| Median (years) | 61 | 60 | 60 |
| Min, Max (years) | 19, 89 | 19, 92 | 19, 92 |
| Age Group, n (%) | |||
| >=17 | 1976 (61) | 2055 (63) | 4031 (62) |
| >=65 years | 1265 (39) | 1209 (37) | 2474 (38) |
| >=75 years | 369 (11.4) | 353 (10.8) | 722 (11.1) |
| Race, n (%) | |||
| White | 2879 (88.8) | 2892 (88.6) | 5771 (88.7) |
| Black or African American | 32 (1) | 43 (1.3) | 75 (1.2) |
| Asian | 268 (8.3) | 264 (8.1) | 532 (8.2) |
| Other | 62 (1.9) | 65 (2) | 127 (2) |
| Region* | |||
| West | 499 (15.4) | 509 (15.6) | 1008 (15.5) |
| East | 2105 (64.9) | 2138 (65.5) | 4243 (65.2) |
| Asia | 261 (8.1) | 259 (7.9) | 520 (8) |
| South America | 376 (11.6) | 358 (11) | 734 (11.3) |
*West included Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Netherlands, Norway, Portugal, Spain, Sweden, Turkey, United Kingdom; East included Bulgaria, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Romania, Russia, Slovakia, Slovenia, Ukraine; Asia included China, Hong King, India, Korea, Malaysia; South America included Argentina, Brazil, Chile.
Source: From Company’s Clinical Trial Data
How were the trials designed?
The trial enrolled patients with certain types of worsening heart failure. Patients were randomly assigned to receive CORLANOR or a placebo pill. Neither the patients nor the health care professionals knew which patients were taking CORLANOR and which ones were taking the placebo pill until after the trial was complete.
The trial measured the timing of two possible events:
- the first time a patient needed to be hospitalized for worsening heart failure, or
- death of a patient that was considered to be related to heart disease
How were the trials designed?
The pivotal trial was a randomized, double-blind trial comparing CORLANOR and placebo in adult patients with stable New York Heart Association class II to IV heart failure, left ventricular ejection fraction ≤ 35%, and resting heart rate ≥ 70 beats per minute (bpm). Patients had to have been clinically stable for at least 4 weeks on an optimized clinical regimen. The regimen included maximally tolerated doses of beta-blockers, ACE inhibitors or Angiotensin Receptor Blockers in most cases, and spironolactone or other diuretics, so that fluid retention and symptoms of congestion were minimized. Patients had to have been hospitalized for heart failure within 12 months prior to study entry.
All subjects were initiated on CORLANOR 5 mg or matching placebo twice daily and the dose was increased to 7.5 mg twice daily or decreased to 2.5 mg twice daily to maintain the resting heart rate between 50 and 60 bpm, as tolerated. The primary endpoint was a composite of the first occurrence of either hospitalization for worsening heart failure or cardiovascular death.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION


