Drug Trials Snapshots: ARISTADA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ARISTADA Prescribing Information for complete information.
ARISTADA (aripiprazole laurixil)
(air-is-TAH-dah)
Alkermes Inc.
Approval date: October 5, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ARISTADA is a drug used for the treatment of schizophrenia.
Schizophrenia is a brain disorder characterized by hearing voices, believing other people are reading one’s mind or controlling one’s thoughts, and being suspicious or withdrawn.
How is this drug used?
ARISTADA is an injection given into the muscle of the arm or buttock by a healthcare provider once a month to once every 6 weeks.
What are the benefits of this drug?
ARISTADA improved symptoms of schizophrenia.
What are the benefits of this drug?
The table below summarizes the primary efficacy endpoints. This was based on the mean change in the Positive and Negative Syndrome Scale (PANSS) total score after 12 weeks. The population represents Full analysis set (FAS) and consists of all randomized patients who received at least one injection dose of study drug and had at least one post-randomization efficacy evaluation for PANSS total score.
Table 2. Summary of Efficacy Results (FAS population)
| Trial Number | Treatment Group | Primary Efficacy Measure: PANSS Total Score | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea (95% CI) |
||
| Trial 1 | ARISTADA 441 mgb | 92.6 (10.2) | -20.9 (1.4) | -10.9 (-14.5, -7.3) |
| ARISTADA 882 mgb | 92.0 (10.8) | -21.8 (1.4) | -11.9 (-15.4, -8.3) | |
| Placebo | 93.9 (11.3) | -9.8 (1.4) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiple comparisons.
a Difference (drug minus placebo) in least-squares mean change from baseline.
b Doses that are demonstrated to be effective.
Source: Prescribing Information, Section 14, Table 13
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex and race.
- Sex: ARISTADA worked similarly in men and women.
- Race: ARISTADA worked similarly in all races studied.
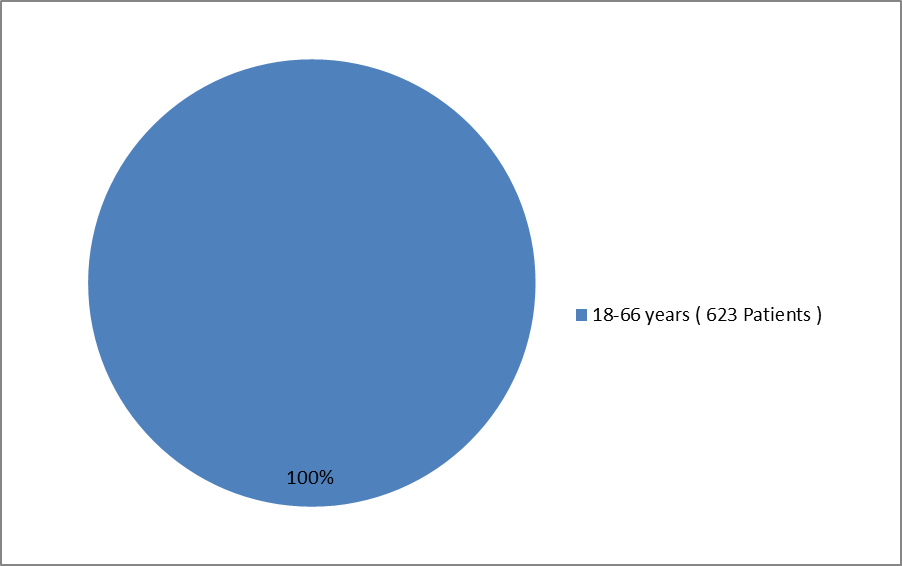
- Age:: Most patients in the trial were 18 to 66 years old. Differences in how well ARISTADA worked in patients below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
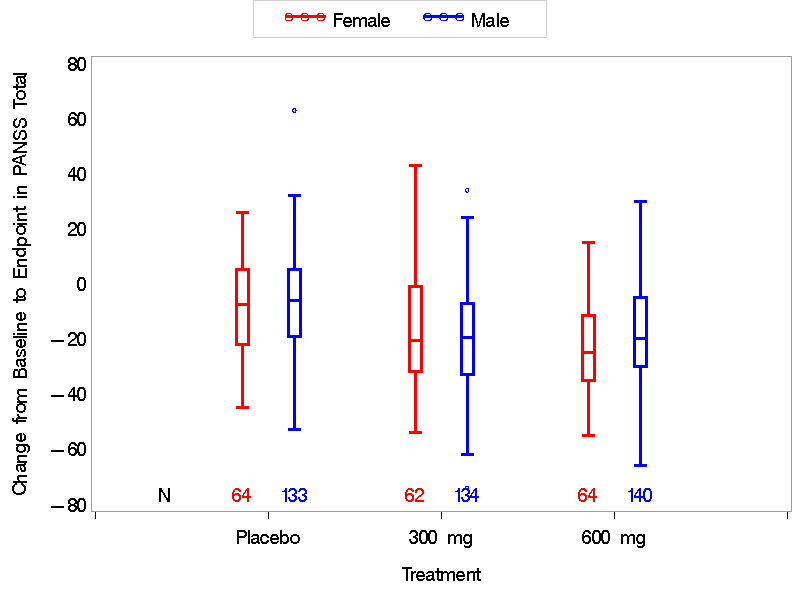
The figures below summarize the responses to ARISTADA by sex and race subgroups for the FAS population which includes all randomized patients who received at least one injection dose of study drug and had at least one post-randomization efficacy evaluation for PANSS total score.
Figure 3. Subgroup Analysis of Primary Endpoint by Sex
Source: FDA Statistical Review
Figure 4. Subgroup Analysis of Primary Endpoint by Race
Source: FDA Statistical Review
What are the possible side effects?
The most common side effect of ARISTADA was akathisia, which is the urge to move constantly.
What are the possible side effects?
The table below summarizes adverse reactions in the Safety population defined as all patients who received at least one injection dose of study drug.
Table 3. Adverse Reactions that Occurred in 2% or more of ARISTADA-Treated Patients and at Greater Incidence than in the Placebo-Treated Patients
| Adverse Reaction | Placebo N=207 (%) |
ARISTADA | |
|---|---|---|---|
| 441 mg N=207 (%) |
882 mg N=208 (%) |
||
| General disorders and administration site conditions | |||
| Injection site pain | 2 | 3 | 4 |
| Investigations | |||
| Increased weight | 1 | 2 | 2 |
| Increased blood creatine phosphokinase | 0 | 2 | 1 |
| Nervous system disorders | |||
| Akathisia | 4 | 11 | 11 |
| Headache | 3 | 3 | 5 |
| Psychiatric disorders | |||
| Insomnia | 2 | 3 | 4 |
| Restlessness | 1 | 3 | 1 |
Source: ARISTADA Prescribing Information, Section 6, Table 10
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex and race.
- Sex: Akathisia (the urge to move constantly) was seen more frequently in men compared to women.
- Race: Akathisia (the urge to move constantly) was seen more frequently in blacks/African Americans compared to whites.
- Age: Most patients in the trial were 18 to 66 years old. Differences in side effects of ARISTADA in patients below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes akathisia in the Safety population defined as all patients who received at least at least one injection dose of study drug.
Table 4. Subgroup Analysis of Akathisia Adverse Event
| Subgroup | ARISTADA 441mg (N=207) n(%) |
ARISTADA 882mg (N=208) n(%) |
Placebo (N=207) n(%) |
|||
|---|---|---|---|---|---|---|
| x (%)** | Total, n | x (%)** | Total, n | x (%)** | Total, n | |
| Akathisia | 24 (11.6) | 207 | 24 (11.5) | 208 | 9 (4.4) | 207 |
| Sex | ||||||
| Male | 20 (14.2) | 141 | 21 (14.7) | 143 | 4 (2.9) | 138 |
| Female | 4 (6.1) | 66 | 3 (4.6) | 65 | 5 (7.3) | 69 |
| Race | ||||||
| White | 9 (9.1) | 99 | 8 (8.2) | 98 | 6 (6.4) | 94 |
| Black or African American | 13 (15.7) | 83 | 14 (17.3) | 81 | 3 (3.6) | 84 |
| Asian | 1 (4.2) | 24 | 2 (7.1) | 28 | 0 (0) | 28 |
| American Indian or Alaska Native | 0 (0) | 0 | 0 (0) | 1 | 0 (0) | 1 |
| Native Hawaiian or Other Pacific Islander | 1 (100) | 1 | 0 (0) | 0 | 0 (0) | 0 |
X (%)**=number and percentage of patients in that subgroup that had akathisia
Source: Company Trial data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved ARISTADA based partly on previous evidence from trials using the oral formulation of aripiprazole. In addition, the benefit or ARISTADA was assessed with evidence from one clinical trial that enrolled 623 patients with schizophrenia. The trial was conducted in the United States, Europe, and Asia.
The figure below summarizes how many men and women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Source: FDA Review Division
The figure and table below summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Demographics by Race
<1%=less than="">
Source: FDA Review Division
Table 1. Demographics of Trial by Race
| Race | Number of patients | Percentage of patients |
|---|---|---|
| White | 291 | 47% |
| Black or African American | 248 | 40% |
| Asian | 81 | 13% |
| American Indian or Alaska Native | 2 | <> |
| Native Hawaiian or Other Pacific Islander | 1 | <> |
<1%=less than="">
Source: FDA Review Division
The figure below summarizes how many patients by age were enrolled in the clinical trial. All subjects were in the 18 to 66 year old range.
Figure 3. Baseline Demographics by Age
Source: FDA Review Division
Who participated in the trials?
The table below summarizes baseline demographics for the randomized population.
Table 5. Baseline Demographics for the Trial
| Subgroup | Placebo (N=208) n (%) |
ARISTADA 441 mg (N=207) n (%) |
ARISTADA 882 mg (N=208) n (%) |
Total (N=623) n (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | 139 (67) | 141 (68) | 143 (67) | 423 (68) |
| Female | 69 (33) | 66 (32) | 65 (31) | 200 (32) |
| Age (years) | ||||
| Mean (SD) | 40 (12) | 40 (10) | 40 (11) | 40 (11) |
| Median | 39 | 39 | 40 | 39 |
| Min-Max | 18-66 | 18-61 | 20-65 | 18-66 |
| Race | ||||
| White | 94 (45) | 99 (48) | 98 (47) | 291 (47) |
| Black or African American | 84 (40) | 83 (40) | 81 (39) | 248 (40) |
| Asian | 29 (14) | 24 (12) | 28 (14) | 81 (13) |
| American Indian or Alaska Native | 1 (<> | 0 | 1 (<> | 2 (<> |
| Native Hawaiian or Other Pacific Islander | 0 | 1 (<> | 0 | 1 (<> |
| Region | ||||
| United States | 102 (49) | 103(50) | 102 (49) | 307 (49) |
| Europe | 80 (39) | 81 (39) | 78 (38) | 239 (39) |
| Asia | 26 (13) | 23 (11) | 28 (14) | 77 (12) |
Source: Review Division
How were the trials designed?
There was one trial that evaluated the benefit and side effects of ARISTADA. In the trial, patients were randomly assigned to receive either ARISTADA or placebo. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The trial lasted 12 weeks, and measured overall improvement in the symptoms of schizophrenia at the end of the trial.
How were the trials designed?
ARISTADA was studied in a 12-week, randomized, double-blind, placebo controlled, fixed-dose trial in adult patients with acutely-relapsed schizophrenia who met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision, (DSM IV TR) criteria.
ARISTADA or placebo injections were administered on Days 1, 29, and 57. Patients also received an oral study drug (aripiprazole or placebo) daily on Days 1 through 21.
The primary endpoint was the change from baseline to Week 12 in Positive and Negative Syndrome Scale (PANSS) total score.
The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 to 210.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION