Drug Trials Snapshots: PORTRAZZA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the PORTRAZZA Prescribing Information for complete information.
PORTRAZZA (necitumumab)
por-tra-zuh
Eli Lilly
Approval date: November 24, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PORTRAZZA is a drug used to treat a type of lung cancer called squamous non-small cell lung cancer (NSCLC) that has become advanced (metastatic). It is to be used in combination with two other cancer drugs, gemcitabine and cisplatin, in patients who have not previously received medication to treat advanced lung cancer.
PORTRAZZA is not indicated for treatment of non-squamous non-small cell lung cancer.
How is this drug used?
PORTRAZZA is given by a healthcare provider as an infusion into a vein, according to a specific schedule.
What are the benefits of this drug?
Patients taking PORTRAZZA plus gemcitabine and cisplatin lived longer on average (11.5 months) compared to those only taking gemcitabine and cisplatin (9.9 months).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table and figure below summarize efficacy results for the clinical trial.
Table 3: Efficacy Results for Metastatic Squamous Non-Small Cell Lung Cancer
| PORTRAZZA PLUS GEMCITABINE AND CISPLATIN N=545 | GEMCITABINE AND CISPLATIN N=548 | |
|---|---|---|
| Overall Survival | ||
| Number of deaths (%) | 418 (77%) | 442 (81%) |
| Median – months (95% CI)a | 11.5 (10.4, 12.6) | 9.9 (8.9, 11.1) |
| Stratified Hazard Ratio (95% CI) | 0.84 (0.74, 0.96) | |
| Stratified Log-rank p-value | 0.01 | |
| Progression-Free Survivalb | ||
| Number of events (%) | 431 (79%) | 417 (76%) |
| Median – months (95% CI) | 5.7 (5.6, 6.0) | 5.5 (4.8, 5.6) |
| Stratified Hazard Ratio (95% CI) | 0.85 (0.74, 0.98) | |
| Stratified Log-rank p-value | 0.02 | |
aAbbreviations: CI = confidence interval
bInvestigator assessed
PORTRAZZA Prescribing Information
Figure 4. Kaplan-Meier Curves of Overall Survival in Patients with Metastatic Squamous Non-Small Cell Lung Cancer

PORTRAZZA Prescribing Information
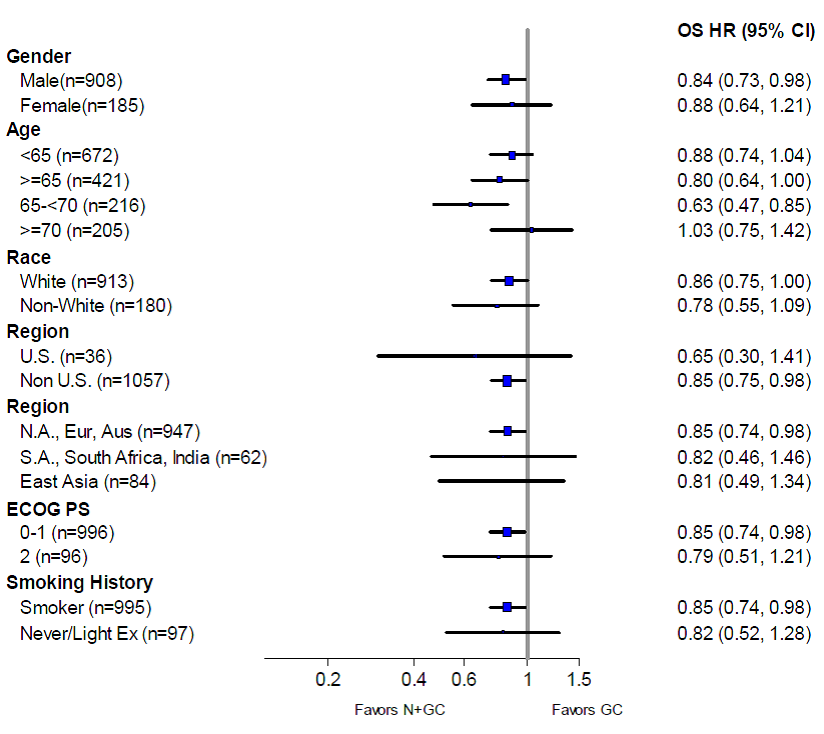
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: PORTRAZZA worked similarly in men and women.
- Race: The majority of patients in the clinical trial were white. Differences in response to PORTRAZZA among races could not be determined.
- Age: PORTRAZZA worked better in patients below 70 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes overall survival by subgroup.
Table 3. Subgroup Analysis of Overall Survival Intent to Treat Population
Clinical Trial Data
What are the possible side effects?
The most common side effects of PORTRAZZA are skin rash and magnesium deficiency (hypomagnesemia), which can cause muscular weakness, seizure, irregular heartbeats, and can be fatal.
Serious side effects of PORTRAZZA include increased risk of cardiac arrest, magnesium deficiency, blood clots in veins and arteries, skin rashes , reactions related to the infusion of the drug through the vein, toxicity to a fetus when given to a pregnant woman, and increased toxicity and increased death in patients with non-squamous NSCLC.
PORTRAZZA increases the risk of clotting events in blood vessels (including venous thromboembolism). Venous thromboembolism is the formation of blood clots in the vein. When a clot forms in a deep vein, usually in the leg, it is called a deep vein thrombosis or DVT. If that clot breaks loose and travels to the lungs, it is called a pulmonary embolism.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions, including electrolyte abnormalities, in the clinical trial.
Table 4. Adverse Reactions Occurring at Incidence Rate ≥5% All Grades or a ≥2% Grade 3-4 Difference Between Arms in Patients Receiving PORTRAZZA in Study 1
| Adverse Reactions (MedDRA) System Organ Class | PORTRAZZA Plus Gemcitabine and Cisplatin N=538 (%) | Gemcitabine and cisplatin N=541 (%) | ||
|---|---|---|---|---|
| All Grades (Frequency %) | Grade 3-4 (Frequency %) | All Grades (Frequency %) | Grade 3-4 (Frequency %) | |
| Skin and Subcutaneous Tissue Disorders | ||||
| Rash | 44 | 4 | 6 | 0.2 |
| Dermatitis Acneiform | 15 | 1 | 0.6 | 0 |
| Acne | 9 | 0.4 | 0.6 | 0 |
| Pruritus | 7 | 0.2 | 0.9 | 0.2 |
| Dry Skin | 7 | 0 | 1 | 0 |
| Skin fissures | 5 | 0.4 | 0 | 0 |
| Gastrointestinal Disorders | ||||
| Vomiting | 29 | 3 | 25 | 0.9 |
| Diarrhea | 16 | 2 | 11 | 1 |
| Stomatitis | 11 | 1 | 6 | 0.6 |
| Investigations | ||||
| Weight decreased | 13 | 0.7 | 6 | 0.6 |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Hemoptysis | 10 | 1 | 5 | 0.9 |
| Pulmonary embolisma | 5 | 4 | 2 | 2 |
| Nervous System Disorders | ||||
| Headache | 11 | 0 | 6 | 0.4 |
| Vascular Disorders | ||||
| Venous Thromboembolic Events (VTE)b | 9 | 5 | 5 | 3 |
| Infections and Infestations | ||||
| Paronychia | 7 | 0.4 | 0.2 | 0 |
| Eye Disorders | ||||
| Conjunctivitisc | 7 | 0.4 | 2 | 0 |
a Pulmonary embolism is also included in the composite term venous thromboembolic events under system organ class vascular disorders.
b VTE is a composite term which includes: pulmonary embolism, deep vein thrombosis, thrombosis, mesenteric veins thrombosis, pulmonary artery thrombosis, pulmonary venous thrombosis, venous thrombosis limb, axillary vein thrombosis, thrombophlebitis, thrombosis in device, vena cava thrombosis, venous thrombosis, subclavian vein thrombosis, superior vena cava syndrome, and thrombophlebitis superficial.
c Conjunctivitis is a composite term that includes conjunctivitis, eye irritation, vision blurred, conjunctivitis bacterial, dry eye, visual acuity reduced, blepharitis, allergic blepharitis, conjunctiva hemorrhage, eye infection, eye pain, lacrimation increased, ocular hyperemia, Sjogren’s syndrome, visual impairment, and eye pruritus.
PORTRAZZA Prescribing Information
Table 5. Thromboembolic Events by Age Group
| 70> | ≥70 | |||||||
|---|---|---|---|---|---|---|---|---|
| GC+N N=432 n (%) | GC N=444 n (%) | GC+N N=106 n (%) | GC N=444 n (%) | |||||
| Any Grade | Gr ≥3 | Any Grade | Gr ≥3 | Any Grade | Gr≥3 | Any Grade | Gr ≥3 | |
| Arterial thromboembolic events | 23 (5.3) | 15 (3.5) | 15 (3.4) | 7 (1.6) | 6 (5.7) | 6 (5.7) | 6 (6.2) | 4 (4.1) |
| Venous thromboembolic events | 36 (8.3) | 21 (4.9) | 25 (5.6) | 11 (2.5) | 13 (12.3) | 6 (5.7) | 4 (4.1) | 3 (3.1) |
GC=Gemcitabine-Cisplatin
GC+N= Gemcitabine-Cisplatin plus Necitumumab
Gr=grade
Clinical Trial Data
Table 6. Electrolyte Abnormalities according to Laboratory Assessment at Incidence Rate >10% and a >2% Difference between Arms in Patients Receiving PORTRAZZA in Study 1a
| LABORATORY PARAMETER | PORTRAZZA Plus Gemcitabine and Cisplatin N=538 | Gemcitabine and cisplatin N=541 | ||||
|---|---|---|---|---|---|---|
| Na | All Grades (Frequency %) | Grade 3 or 4 (Frequency %) | Na | All Grades (Frequency %) | Grade 3 or 4 (Frequency %) | |
| Hypomagnesemia | 461 | 83 | 20 | 457 | 70 | 7 |
| Hypokalemia | 505 | 28 | 5 | 505 | 18 | 3 |
| Hypocalcemia | 502 | 45 | 6 | 499 | 30 | 2 |
| Hypocalcemia (albumin corrected) | 477 | 36 | 4 | 480 | 23 | 2 |
| Hypophosphatemia | 462 | 31 | 8 | 454 | 23 | 6 |
aOnly patients with baseline and at least one post-baseline result are included.
PORTRAZZA Prescribing Information
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of side effects was similar in men and women.
- Race: The majority patients in the clinical trial were white. Differences in side effects among races could not be determined.
- Age: The risk of overall side effects was similar in patients below and above 65 years of age. The risk of venous thromboembolism was higher in patients age 70 and over compared to those who were younger than age 70.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events during the clinical trial by subgroup.
Table 7. Subgroup Analysis of Treatment-Emergent Adverse Events (Safety Population)
| Gemcitabine-Cisplatin Chemotherapy Plus Necitumumab | Gemcitabine-Cisplatin Chemotherapy Alone | |||||
|---|---|---|---|---|---|---|
| Demographic Subgroup | n | % | Total, N | n | (%) | Total, N |
| Any TEAEs | ||||||
| Sex | ||||||
| Male | 441 | 97.6 | 452 | 443 | 99.3 | 446 |
| Female | 88 | 98.9 | 89 | 90 | 97.8 | 92 |
| Age Group | ||||||
| 17> | 0 | 0 | ||||
| >=17 - 65> | 325 | 97.3 | 334 | 325 | 99.1 | 328 |
| >= 65 years | 204 | 98.6 | 207 | 208 | 99 | 210 |
| >= 75 years | 19 | 100 | 19 | 24 | 96 | 25 |
| Race | ||||||
| White | 442 | 97.8 | 452 | 447 | 98.9 | 452 |
| Black or African American | 6 | 100 | 6 | 5 | 100 | 5 |
| Asian | 38 | 95 | 40 | 41 | 100 | 41 |
| American Indian or Alaska Native | 0 | 1 | 100 | 1 | ||
| Native Hawaiian or Other Pacific Islander | 1 | 100 | 1 | 0 | ||
| Other | 42 | 100 | 42 | 39 | 100 | 39 |
| Ethnicity | ||||||
| Hispanic or Latino | 54 | 100 | 54 | 54 | 98.2 | 55 |
| Not Hispanic or Latino | 473 | 97.5 | 485 | 478 | 99.2 | 482 |
| Missing | 2 | 100 | 2 | 1 | 100 | 1 |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved PORTRAZZA based on evidence from a clinical trial of 1093 patients. The trials were conducted at 184 clinical sites across North America (including the United States), South America, Europe, Australia, Africa, and Asia.
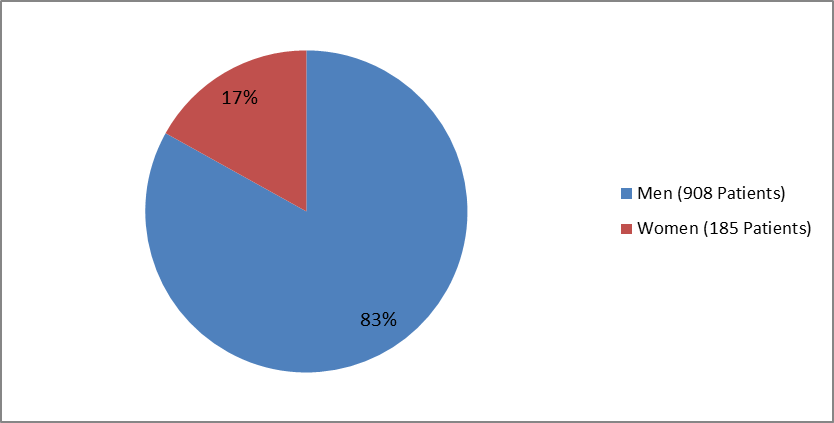
Figure 1 summarizes how many men and women were enrolled in the clinical trial.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
Figure 2 and Table 1 summarize how many patients by race were enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 913 | 83% |
| Black or African American | 11 | 1% |
| Asian | 85 | 8% |
| American Indian or Alaska Native | 1 | > |
| Native Hawaiian or Other Pacific Islander | 1 | > |
| Other | 82 | 8% |
Clinical Trial Data
The figure below summarizes how many patients by age were enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trial.
Table 8. Baseline Demographics of Patients in the Clinical Trial
| Comparator/Control Gemcitabine-Cisplatin Chemotherapy Alone (N=548) | Treatment Group Gemcitabine-Cisplatin Chemotherapy Plus Necitumumab (N=545) | Total (N=1093) | |||
|---|---|---|---|---|---|
| Demographic Parameters | n | % | n | % | n (%) |
| Sex | |||||
| Male | 458 | (83.6) | 450 | (82.6) | 908 (83.1) |
| Female | 90 | (16.4) | 95 | (17.4) | 185 (16.9) |
| Age | |||||
| Mean years (SD) | 61.7 | (8.20) | 62.0 | (8.32) | 61.8 (8.26) |
| Median (years) | 62.0 | 62.0 | 62.0 | ||
| Min, Max (years) | 32, 86 | 32, 84 | 32, 86 | ||
| Age Group | |||||
| >=17 - 65> | 340 | (62.0) | 332 | (60.9) | 672 (61.5) |
| >=65 years | 208 | (38.0) | 213 | (39.1) | 421 (38.5) |
| >= 75 years | 19 | (3.5) | 25 | (4.6) | 44 (4.0) |
| Race | |||||
| White | 456 | (83.2) | 457 | (83.9) | 913 (83.5) |
| Black or African American | 6 | (1.1) | 5 | (0.9) | 11 (1.0) |
| Asian | 42 | (7.7) | 43 | (7.9) | 85 (7.8) |
| American Indian or Alaska Native | 0 | 1 | (0.2) | 1 (0.1) | |
| Native Hawaiian or Other Pacific Islander | 1 | (0.2) | 0 | 1 (0.1) | |
| Other | 43 | (7.8) | 39 | (7.2) | 82 (7.5) |
Clinical Trial Data
How were the trials designed?
The benefit and side effects of PORTRAZZA were evaluated in a clinical trial of patients with advanced squamous Non-Small Cell Lung Cancer (NSCLC). The patients were randomly assigned to receive gemcitabine and cisplatin, either with or without PORTAZZA. Both patients and healthcare workers knew which treatment was being administered. The efficacy of PORTRAZZA was evaluated by measuring how long patients survived.
How were the trials designed?
The pivotal trial was a randomized, multi-center open-label, controlled trial conducted in patients receiving gemcitabine and cisplatin first-line chemotherapy for metastatic squamous NSCLC. Patients were randomized (1:1) to receive PORTRAZZA plus gemcitabine and cisplatin or gemcitabine and cisplatin alone. Stratification factors were ECOG performance status (0, 1 versus 2) and geographic region (North America, Europe, and Australia versus South America, South Africa, and India versus Eastern Asia).
Gemcitabine (1250 mg/m2, Days 1 and 8) plus cisplatin (75 mg/m2, Day 1) were administered every 3 weeks (1 cycle) for a maximum of 6 cycles in the absence of disease progression or unacceptable toxicity. PORTRAZZA (800 mg by intravenous infusion on Days 1 and 8 of each 3-week cycle) was administered prior to gemcitabine and cisplatin.
Patients demonstrating at least stable disease on PORTRAZZA plus gemcitabine and cisplatin were to continue PORTRAZZA as a single agent in the absence of disease progression or unacceptable toxicity after completion of 6 planned courses of chemotherapy or if chemotherapy was discontinued for toxicity. The main outcome measure was overall survival (OS). Investigator-assessed progression-free survival (PFS) and overall response rate (ORR) were also assessed.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION