Drug Trials Snapshots: NUCALA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the NUCALA Prescribing Information for complete information.
NUCALA (mepolizumab)
(new-ka′la)
GlaxoSmithKline LLC.
Approval date: November 4, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NUCALA is a drug for the treatment of a specific group of patients with severe asthma (those with an eosinophilic phenotype) who are 12 years and older and whose asthma is not well controlled with current medications. NUCALA is to be used in addition to asthma maintenance medications.
How is this drug used?
NUCALA is given once every four weeks by injection under the skin (subcutaneously) by a health care professional into the upper arm, thigh, or abdomen.
What are the benefits of this drug?
Patients who received NUCALA:
- Had fewer asthma attacks that required a stay in the hospital and/or a visit to the emergency room
- Had greater reduction in their daily maintenance dose of oral corticosteroids
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results on exacerbation rates endpoints for the Trials 1 and 2. Included are the results from dose ranging (not approved dose of 75 mg IV).
Table 2. Rate of Exacerbations in Trials 1 and 2 (Intent-to-Treat Population)
| Trial | Treatment | Exacerbations per Year | ||
|---|---|---|---|---|
| Rate | Difference | Rate Ratio (95% CI) | ||
| All Exacerbations | ||||
| Exacerbations requiring hospitalization/emergency room visit | ||||
| Exacerbations requiring hospitalization | ||||
| Trial 1 | Placebo (n = 155) | 2.40 | ||
| Mepolizumab 75 mg IV (n = 153) | 1.24 | 1.16 | 0.52 (0.39, 0.69) | |
| Trial 2 | Placebo (n = 191) | 1.74 | ||
| Mepolizumab 75 mg IV (n = 191) | 0.93 | 0.81 | 0.53 (0.40, 0.72) | |
| NUCALA 100 mg SC (n = 194) | 0.83 | 0.91 | 0.47 (0.35, 0.64) | |
| Trial 1 | Placebo (n = 155) | 0.43 | ||
| Mepolizumab 75 mg IV (n = 153) | 0.17 | 0.26 | 0.40 (0.19, 0.81) | |
| Trial 2 | Placebo (n = 191) | 0.20 | ||
| Mepolizumab 75 mg IV (n = 191) | 0.14 | 0.06 | 0.68 (0.33, 1.41) | |
| NUCALA 100 mg SC (n = 194) | 0.08 | 0.12 | 0.39 (0.18, 0.83) | |
| Trial 1 | Placebo (n = 155) | 0.18 | ||
| Mepolizumab 75 mg IV (n = 153) | 0.11 | 0.07 | 0.61 (0.28, 1.33) | |
| Trial 2 | Placebo (n = 191) | 0.10 | ||
| Mepolizumab 75 mg IV (n = 191) | 0.06 | 0.04 | 0.61 (0.23, 1.66) | |
| 0.03 | 0.07 | 0.31 (0.11,0.91 | |
Efficacy results for oral corticosteroid reduction (Trial 3)
Sixteen (23%) patients in the group receiving NUCALA versus 7 (11%) in the placebo group had a 90% to 100% reduction in their oral corticosteroid dose. Twenty-five (36%) patients in the group receiving NUCALA versus 37 (56%) in the placebo group were classified as having no improvement for oral corticosteroid dose. Additionally, 54% of patients treated with NUCALA achieved at least a 50% reduction in the daily prednisone dose compared with 33% of subjects treated with placebo (95% CI for difference: 4%, 37%).
NUCALA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: NUCALA worked similarly in men and women.
- Race: NUCALA worked similarly in Black or African American, White, and Asian patients.
- Age: NUCALA worked similarly in all age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup analyses of asthma exacerbations (combining all doses and Trials 1 and 2) are presented in the figure below.
Figure 4. Asthma Exacerbation Rate Ratio by Age, Sex, Race, and Ethnicity (Trials 1 and 2)
P-value for statistical test measuring whether the treatment effect differs across subgroups (i.e., p-value for test of treatment-by-subgroup interaction) for sex p= 0.239, age p=0.129, race p=0.152, and ethnicity p=0.114.
Source: FDA Statistical Review for Drug Snapshot (PDF - 239KB)
What are the possible side effects?
The most common side effects of NUCALA are injection site reactions, back pain, and weakness (fatigue).
NUZALA may cause serious reactions including allergic reactions (swelling of the face, mouth, and tongue; fainting, dizziness, or lightheadedness; hives; breathing problems and rash) and Herpes zoster infections. Herpes zoster is the virus that causes shingles.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes the adverse reactions that occurred in patients treated with NUCALA in Trials 2 and 3.
Table 3 . Adverse Reactions with NUCALA with Greater than or Equal to 3% Incidence and More Common than Placebo in Subjects with Asthma (Trials 2 and 3)
| Adverse Reaction | NUCALA (n = 263) % | Placebo (n = 257) % |
|---|---|---|
| Headache | 19 | 18 |
| Injection site reaction | 8 | 3 |
| Back pain | 5 | 4 |
| Fatigue | 5 | 4 |
| Influenza | 3 | 2 |
| Urinary tract infection | 3 | 2 |
| Abdominal pain upper | 3 | 2 |
| Pruritus | 3 | 2 |
| Eczema | 3 | <> |
| Muscle spasms | 3 | <> |
NUCALA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effect was similar among Black or African American, White, and Asian patients.
- Age: The majority of patients in the trials were below age 65. Differences in side effects between patients above and below age 65 could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events by subgroup. Included are the results for all doses.
Table 4. Adverse Events by Subgroups
| Demographic Parameters | Placebo N=412 | Mepolizumab | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NUCALA 100 SC* N=263 | 75 IV N=344 | 250 IV N=152 | 750 IV N=156 | All doses N=915 | |||||
| Sex | |||||||||
| Age | |||||||||
| Race | |||||||||
| Any event, n (%) | 195 (83) | 132 (83) | 180 (86) | 79 (85) | 77 (83) | 468 (84) | |||
| Male, n | 178 | 103 | 135 | 59 | 63 | 360 | |||
| Any event, n(%) | 143 (80) | 77 (75) | 107 (79) | 45 (76) | 45 (71) | 274 (76) | |||
| 12-17 years, n | 9 | 9 | 9 | 1 | 0 | 28 | |||
| Any event, n (%) | 6 (67) | 7 (78) | 9 (100) | 1 (100) | 17 (89) | 23 (82) | |||
| 18 – 64 years, n | 366 | 216 | 302 | 143 | 153 | 814 | |||
| Any event, n(%) | 298 (81) | 172 (80) | 249 (82) | 116 (81) | 119 (78) | 656 (81) | |||
| ≥ 65 years, n | 37 | 38 | 33 | 8 | 3 | 82 | |||
| Any event n (%) | 34 (92) | 30 (79) | 29 (88) | 7 (88) | 3 (100) | 69 (84) | |||
| African American, n | 9 | 7 | 11 | 7 | 5 | 30 | |||
| Any event n (%) | 7 (78) | 6 (86) | 11 (100) | 7 (100) | 4 (80) | 28 (93) | |||
| White, n | 349 | 219 | 288 | 136 | 140 | 783 | |||
| Any event n (%) | 281 (81) | 173 (79) | 237 (82) | 108 (79) | 107 (76) | 625 (80) | |||
| Asian, n | 49 | 35 | 43 | 7 | 10 | 95 | |||
| Any event n (%) | 45 (92) | 29 (83) | 37 (86) | 7 (100) | 10 (100) | 83 (87) | |||
| Other, n | 5 | 2 | 2 | 2 | 1 | 7 | |||
| Any event n (%) | 5 (100) | 1 (50) | 2 (100) | 2 (100) | 1 (100) | 6 (86) | |||
*Approved dose
FDA Clinical review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved NUCALA based on evidence from 3 clinical trials of 1327 patients with severe asthma. The trials were conducted in countries spanning North America, South America, Europe, Asia, and Australia.
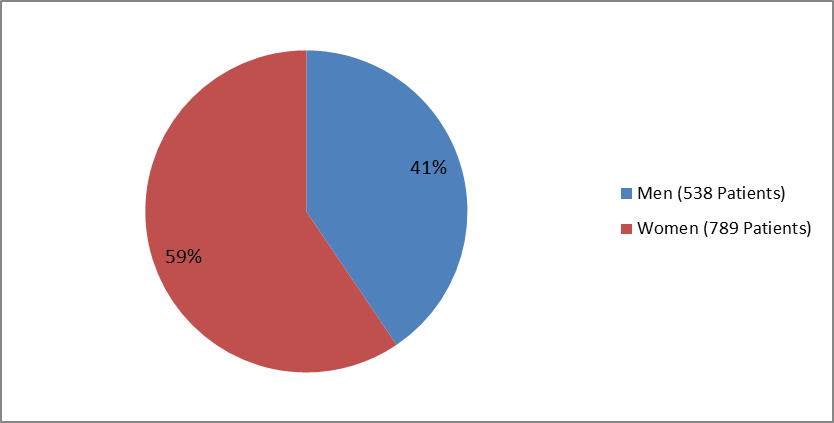
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial data
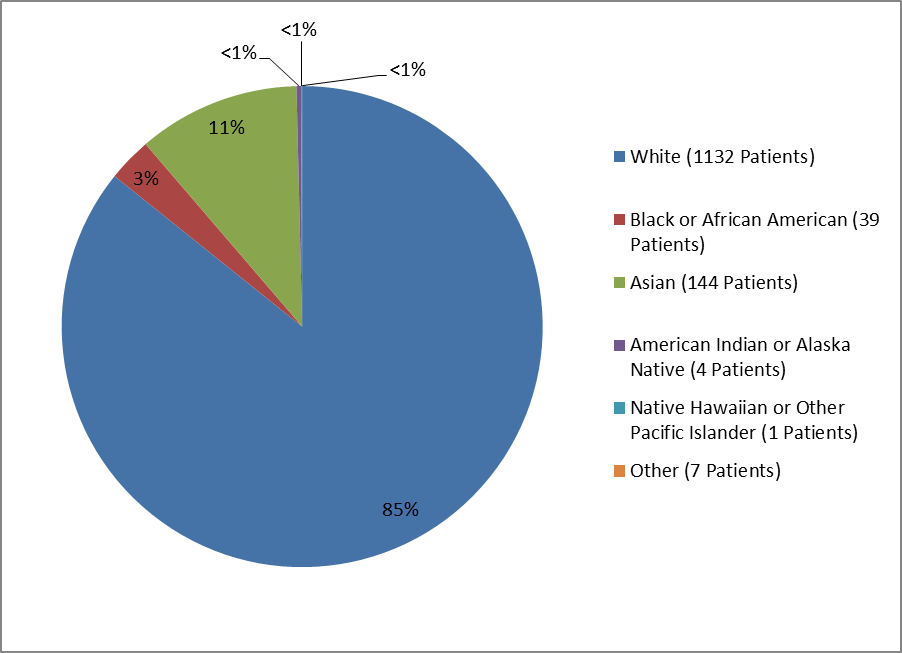
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Demographics of Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1132 | 85% |
| Black or African American | 39 | 3% |
| Asian | 144 | 11% |
| American Indian or Alaska Native | 4 | less than 1% |
| Native Hawaiian or Other Pacific Islander | 1 | less than 1% |
| Other | 7 | less than 1% |
Clinical trial data
Figure 3 summarizes the percentage of patients by age group.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 5. Baseline Demographics for Pooled Safety Population for Trials 1-3 (N=1327)
| Demographic Parameters | Trial 1 (N=616) | Trial 2 (N=576) | Trial 3 (N=135) | Pooled Trials (N=1327) | ||
|---|---|---|---|---|---|---|
| Sex | ||||||
| Age | ||||||
| Age Group | ||||||
| Race | ||||||
| Region | ||||||
| Men | 229 (37%) | 248 (43%) | 61 (45%) | 538 (41%) | ||
| Women | 387 (63%) | 328 (57%) | 74 (55%) | 789 (59%) | ||
| Mean years (SD) | 48.6 (11.28) | 50.1 (14.28) | 49.9 (12.34) | 49.4 (12.78) | ||
| Median (years) | 50 | 52 | 51 | 51 | ||
| Min, Max (years) | 15, 74 | 12, 82 | 16, 74 | 12, 82 | ||
| 12-17 years | 1 (<> | 25 (4%) | 2 (1%) | 28 (2%) | ||
| 18-64 years | 590 (96%) | 471 (82%) | 119 (88%) | 1180 (89%) | ||
| 65 and above | 25 (4%) | 80 (14%) | 14 (10%) | 119 (9%) | ||
| White | 554 (90%) | 450 (78%) | 128 (95%) | 1132 (85%) | ||
| African American or Black | 23 (4%) | 16 (3%) | 0 | 39 (3%) | ||
| Asian | 35 (6%) | 106 (18%) | 3 (2%) | 144 (11%) | ||
| American Indian or Alaska Native | 2 (<> | 1 (<> | 1 (<> | 4 (<> | ||
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 1 (<> | 1 (<> | ||
| Other | 2 (<> | 3 (<> | 2 (1%) | 7 (<> | ||
| United States | 78 (13%) | 67 (12%) | 7 (5%) | 152 (11%) | ||
| European Union* | 280 (45%) | 270 (47%) | 98 (73%) | 648 (49%) | ||
| Asia** | 24 (4%) | 95 (16%) | 0 | 119 (9%) | ||
| Other*** | 234 (38%) | 144 (25%) | 30 (22%) | 408 (31%) | ||
*European Union includes: Belgium, Czech Republic, France, Germany, Italy, Netherlands, Poland, Romania, Spain, United Kingdom
**Asia includes: Japan, Korea
***Other includes: Argentina, Australia, Canada, Chile, Mexico, Russia, Ukraine
Clinical trial data
How were the trials designed?
There were three trials that evaluated the benefits and side effects of NUCALA. NUCALA was given every 4 weeks in all 3 trials in addition to a patient’s current treatment for asthma. All patients continued their usual asthma therapy throughout the duration of the trials.
In each trial, patients were randomly assigned to receive NUCALA or placebo injections. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
How were the trials designed?
NUCALA trials include 3 double-blind, randomized, placebo-controlled trials: 1 dose-ranging and exacerbation trial (Trial 1) and 2 confirmatory trials (Trials 2 and 3). In trial 1, trial participants were required to have elevated blood eosinophils or another marker of an eosinophilic phenotype of severe asthma. In trials 2 and 3 trials participants were only required to have elevated blood eosinophils.
NUCALA was administered every 4 weeks in all 3 trials as add-on to background treatment.
The primary endpoint for Trials 1 and 2 was the frequency of exacerbations defined as worsening of asthma requiring use of oral/systemic corticosteroids and/or hospitalization and/or emergency department visits.
The primary endpoint for Trial 3 was the percent reduction of oral corticosteroid dose during Weeks 20 to 24 compared with baseline dose, while maintaining asthma control.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.