Drug Trials Snapshots: IMFINZI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to IMFINZI Prescribing Information for complete information.
IMFINZI (durvalumab)
im-FIN-zee
AstraZeneca Pharmaceuticals
Approval date: May 1, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
IMFINZI is a drug for the treatment of a type of bladder and urinary tract cancer called urothelial carcinoma. IMFINZI may be used when:
- cancer has spread (locally advanced or metastatic urothelial carcinoma) and,
- chemotherapy that contains platinum did not work or is no longer working.
IMFINZI was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
How is this drug used?
IMFINZI is given every two weeks by a healthcare provider using a needle placed in a vein (known as intravenous infusion). It takes about 60 minutes to receive the full dose of IMFINZI.
What are the benefits of this drug?
Seventeen percent of 182 patients who were treated with IMFINZI responded to the treatment with complete or partial shrinkage of their cancer. Forty-five percent of responding patients maintained their response for more than 6 months and some for 12 months or longer.
More trials are ongoing to confirm the clinical benefit of IMFINZI.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results based on objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 as measured by blinded independent central review (BICR) and duration of response (DoR).
Table 2. Efficacy Results for Trial 1
| All Patients N = 182 |
PD-L1 High N = 95 |
PD-L1 Low/Negative N = 73 |
PD-L1 NE N = 14 |
|
|---|---|---|---|---|
| Objective Response Rate by BICR n (%) (95% CI) |
31 (17.0%) (11.9, 23.3) |
25 (26.3%) (17.8, 36.4) |
3 (4.1%) (0.9, 11.5) |
3 (21.4%) (4.7, 50.8) |
| Complete Response | 5 | 3 | 1 | 1 |
| Partial Response | 26 | 22 | 2 | 2 |
| Median Duration of Response months (range) | NR (0.9+, 19.9+) |
NR (0.9+, 19.9+) |
12.3 (1.9+, 12.3) |
NR (2.3+, 2.6+) |
BICR = Blinded Independent Central Review; NE = Not Evaluable; NR = Not Reached, + denotes an ongoing response
IMFINZI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: IMFINZI worked similarly in men and women.
- Race: Majority of the patients was White; therefore differences in response to IMFINZI among races could not be determined.
- Age: IMFINZI worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by age, sex and race subgroups. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 3. ORR by PD-L1 status in the Overall Efficacy Population for Various Subgroups
| Subgroup | Overall (N=182) n/N % (95% CI) |
PD-L1 Expression Subgroups | |||
|---|---|---|---|---|---|
| PD-L1 High (N=95) n/N % (95% CI) |
PD-L1 Low/negative (N=73) n/N % (95% CI) |
PD-L1 NE (N=14) n/N % (95% CI) |
|||
| Age | |||||
| <65 (n=70) | 14/70 20% (11.4,31.3) |
12/35 34.3% (19.1, 52.2) |
1/30 3.3% (0.1, 17.2) |
1/5 20% (0.5, 71.6) |
|
| >=65 (n=112) | 17/112 15.2% (9.1, 23.2) |
13/60 21.7% (12.1, 34.2) |
2/43 4.7% (0.6, 15.8) |
2/9 22.2% (2.8, 60) |
|
| Sex | |||||
| Women (n=51) | 5/51 9.8% (3.3, 21.4) |
5/26 19.2% (6.6, 39.4) |
0/22 0% (0, 15.4) |
0/3 0% (0, 70.8) |
|
| Men (n=131) | 26/131 19.8% (13.4, 27.7) |
20/69 29% (18.7, 41.2) |
3/51 5.9% (1.2, 16.2) |
3/11 27.3% (6, 61) |
|
| Race | |||||
| Asian (n=36) | 6/36 16.7% (6.4, 32.8) |
5/15 33.3% (11.8, 61.6) |
0/18 0% (0, 18.5) |
1/3 33.3% (0.8, 90.6) |
|
| Other (n=29) | 8/29 27.6% (12.7, 47.2) |
4/15 26.7% (7.8, 55.1) |
2/11 18.2% (2.3, 51.8) |
2/3 66.7% (9.4, 99.2) |
|
| White (n=117) | 17/117 14.5% (8.7, 22.2) |
16/65 24.6% (14.8, 36.9) |
1/44 2.3% (0.1, 12) |
0/8 0% (0, 36.9) |
|
FDA Review
What are the possible side effects?
IMFINZI can cause immune system to attack normal organs and tissues which may lead to serious side effects including inflammation of the lungs, liver, colon, kidneys and endocrine glands. IMFINZI may cause serious or life threatening infusion reactions and infections.
The most common side effects of IMFINZI are tiredness, muscle or bone pain, constipation, decreased appetite, nausea, swelling of the limbs, and urinary tract infection.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions and laboratory abnormalities that were reported during the trial.
Table 4. Adverse Reactions that Occurred in at Least 10% Patients
| IMFINZI N=182 |
||
|---|---|---|
| Adverse Reaction | All Grades (%) |
Grades 3 - 4 (%) |
| All Adverse Reactions | 96 | 43 |
| Gastrointestinal Disorders | ||
| Constipation | 21 | 1 |
| Nausea | 16 | 2 |
| Abdominal pain1 | 14 | 3 |
| Diarrhea/Colitis | 13 | 1 |
| General Disorders and Administration | ||
| Fatigue2 | 39 | 6 |
| Peripheral edema3 | 15 | 2 |
| Pyrexia/Tumor associated fever | 14 | 1 |
| Infections | ||
| Urinary tract infection4 | 15 | 4 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite/Hypophagia | 19 | 1 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Musculoskeletal pain5 | 24 | 4 |
| Respiratory, Thoracic, and Mediastinal Disorders | ||
| Dyspnea/Exertional Dyspnea | 13 | 2 |
| Cough/Productive Cough | 10 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash6 | 11 | 1 |
1 Includes abdominal pain upper, abdominal pain lower and flank pain
2 Includes asthenia, lethargy, and malaise
3 Includes edema, localized edema, edema peripheral, lymphedema, peripheral swelling, scrotal edema, and scrotal swelling
4 Includes cystitis, candiduria and urosepsis
5 Includes back pain, musculoskeletal chest pain, musculoskeletal pain and discomfort, myalgia, and neck pain
6 Includes dermatitis, dermatitis acneiform, dermatitis psoriasiform, psoriasis, rash maculo-papular, rash pruritic, rash papular, rash pustular, skin toxicity, eczema, erythema, erythema multiforme, rash erythematous, acne, and lichen planus
Table 5. Grade 3-4 Laboratory Abnormalities Worsened from Baseline Occurring in ≥1% Patients in Trial 1
| Laboratory Test | Grade 3 - 4 % |
|---|---|
| Hyponatremia | 12 |
| Lymphopenia | 11 |
| Anemia | 8 |
| Increased alkaline phosphatase | 4 |
| Hypermagnesemia | 4 |
| Hypercalcemia | 3 |
| Hyperglycemia | 3 |
| Increased AST | 2 |
| Increased ALT | 1 |
| Hyperbilirubinemia | 1 |
| Increased creatinine | 1 |
| Neutropenia | 1 |
| Hyperkalemia | 1 |
| Hypokalemia | 1 |
| Hypoalbuminemia | 1 |
IMFINZI Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Majority of the patients was White; therefore the differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Tables below summarize safety results by age and sex subgroups. Subgroup analysis based on race was not performed as the trial population was largely White. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 6. Grade 1-4 Adverse Events in >15% of Patients by Age
| Adverse Events | < 65= years> (N = 70) n (%) |
> 65 years (N = 112) n (%) |
||
|---|---|---|---|---|
| Grade 1-4 | Grade 3-4 | Grade 1-4 | Grade 3-4 | |
| All | 69 (99%) | 40 (57%) | 106 (95%) | 43 (38%) |
| Fatigue | 28 (40%) | 3 (4%) | 43 (38%) | 7 (6%) |
| Decreased Appetite | 16 (23%) | 0 | 19 (17%) | 1 (1%) |
| Nausea | 9 (13%) | 2 (3%) | 19 (17%) | 1 (1%) |
| Constipation | 16 (23%) | 2 (3%) | 23 (21%) | 0 |
| Peripheral Edema | 11 (16%) | 3 (4%) | 15 (13%) | 0 |
| Musculoskeletal Pain | 18 (26%) | 3 (4%) | 25 (22%) | 4 (4%) |
Table 7. Grade 1-4 Adverse Events in > 15% of Patients by Sex
| Adverse Events | Men (N =131) n (%) |
Women (N = 51) n (%) |
||
|---|---|---|---|---|
| Grade 1-4 | Grade 3-4 | Grade 1-4 | Grade 3-4 | |
| All | 128 (98%) | 56 (43%) | 47 (92%) | 27 (53%) |
| Fatigue | 49 (37%) | 6 (5%) | 22 (43%) | 4 (8%) |
| Decreased Appetite | 24 (18%) | 1 (1%) | 11 (22%) | 0 |
| Nausea | 18 (14%) | 1 (1%) | 10 (20%) | 2 (4%) |
| Diarrhea | 27 (21%) | 1 (1%) | 12 (24%) | 1 (2%) |
| Peripheral Edema | 21 (16%) | 1 (1%) | 5 (10%) | 2 (4%) |
| Musculoskeletal Pain | 33 (25%) | 4 (3%) | 10 (20%) | 3 (6%) |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved IMFINZI based on evidence from one clinical trial of 182 patients with advanced bladder and urinary tract cancer. The trial was conducted in the United States, Canada and Europe.
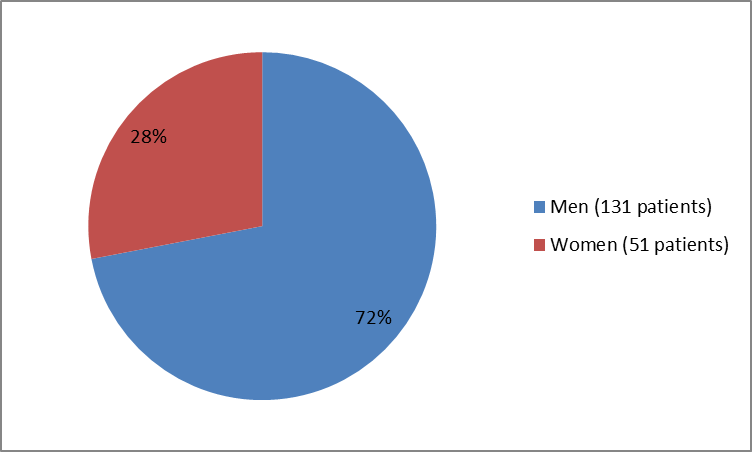
The figure below summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA Review
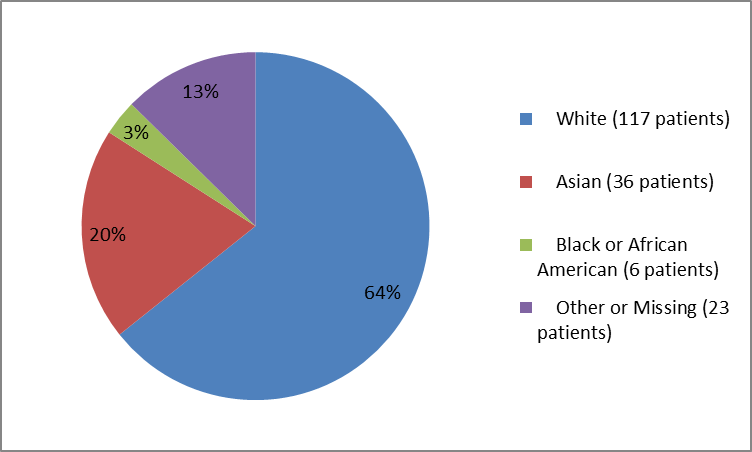
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 117 | 64 |
| Asian | 36 | 20 |
| Black or African American | 6 | 3 |
| Other | 6 | 3 |
| Missing | 17 | 9 |
FDA Review
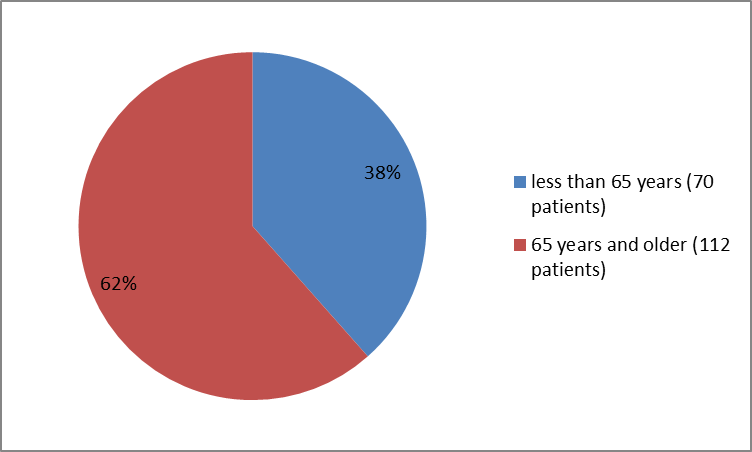
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trial.
Table 8. Baseline Demographics of Patients in the Clinical Trial
| IMFINZI (N=182) n (%) |
||||
|---|---|---|---|---|
| Sex | ||||
| Men | 131 (72) | |||
| Women | 51 (28) | |||
| Age (years) | ||||
| Min, Max | 34, 88 | |||
| Median | 66 | |||
| Age Group | ||||
| <65> | 70 (38) | |||
| ≥65 years | 112 (62) | |||
| Race | ||||
| White | 117 (64) | |||
| Asian | 36 (20) | |||
| Black or African American | 6 (3) | |||
| Other | 6 (3) | |||
| Missing | 17 (9) | |||
| Geographic Region | ||||
| USA | 90 (49) | |||
| All other | 92 (51) | |||
Clinical trial data
How were the trials designed?
The benefit and side effects of IMFINZI were evaluated in one clinical trial of patients with advanced bladder and urinary tract cancer. Patients’ cancer had progressed while receiving or after they received a platinum-based therapy.
In the trial, all patients received treatment with IMFINZI every 2 weeks. The treatment continued for up to 12 months or until either the disease worsened or the patient developed an unacceptable side effect.
The benefit of IMFINZI was evaluated after 12 months by measuring how well the patients responded to IMFINZI and by how long that response lasted.
How were the trials designed?
There was one single-arm, open-label, multicenter clinical trial in patients with histologically confirmed locally advanced or metastatic urothelial carcinoma whose disease had progressed on or after platinum-based therapy. Patients received IMFINZI 10 mg/kg as an intravenous infusion over 60 minutes every 2 weeks for up to 12 months or until unacceptable toxicity or disease progression.
The efficacy outcome measures were confirmed objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 as measured by blinded independent central review and duration of response. The efficacy analysis was conducted at Weeks 6, 12 and 16, then every 8 weeks for the first year and every 12 weeks thereafter.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.