Drug Trials Snapshots: Marcrilen
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the MACRILEN Package Insert for complete information.
MACRILEN (macimorelin acetate)
ma-kri-len
Aeterna Zentaris GMBH
Approval date: December 20, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MACRILEN is a drug for the diagnosis of growth hormone deficiency in adults.
How is this drug used?
MACRILEN is available as granules in a pouch. The granules are dissolved in water by a healthcare professional and given by mouth only once in preparation for a blood test that can help detect growth hormone deficiency. The amount given depends on the patient’s weight.
What are the benefits of this drug?
The results of MACRILEN test may show growth hormone deficiency.
What are the benefits of this drug (results of trials used to assess efficacy)?
The diagnostic performance of the MACRILEN test was established in a trial that compared the outcome of the MACRILEN test with the insulin tolerance test in patients with high, intermediate or low likelihood of having adult growth hormone deficiency (AGHD) and healthy control subjects. The primary efficacy outcomes were negative and positive agreement between the two tests, using prespecified growth hormone threshold of 2.8 ng/mL for MACRILEN and 5.1 ng/mL for the insulin tolerance test. Negative agreement is the proportion of subjects with a negative insulin tolerance test who also have a negative MACRILEN test. Positive agreement is the proportion of subjects with a positive insulin tolerance test who also have a positive MACRILEN test.
Table 2. Diagnostic Outcomes for MACRILEN and the Insulin Tolerance Test in Trial Participants
| All Trial Participants | Insulin Tolerance Test | Total | Positive Agreement: 74% Negative Agreement: 94% Overall Agreement: 84% | ||
|---|---|---|---|---|---|
| + | - | ||||
| MACRILEN | + | 55 | 4 | 59 | |
| - | 19 | 62 | 81 | ||
| Total | 74 | 66 | 140 | ||
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MACRILEN worked similarly in men and women.
- Race: The majority of patients were White. The number of patients of other races were limited; therefore, differences in how the drug worked among other races could not be determined.
- Age: The majority of patients were younger than the age of 65; the number of patients older than 65 years was small; therefore, differences in how the drug worked in older patients could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Figure 4 shows positive and negative agreements between the MACRILEN and insulin tolerance test by sex, race and age category. The small sample size in age and race subgroups does not allow interpretation of differences in outcome.
Figure 4. Positive and Negative Agreement (95% Confidence Interval) Between MACRILEN and Insulin Tolerance Test by Sex, Race and Age
Adapted from FDA Statistics Review
What are the possible side effects?
MACRILEN may cause heart rhythm problems due to change on electric heart beat tracing (ECG) called QTc prolongation and should be avoided in patients receiving other drugs that also cause QTc prolongation.
The most common side effects are change in the sense of taste, headache, diarrhea, nausea and fatigue.
What are the possible side effects (results of trials used to assess safety)?
Table 3 shows the frequency of adverse reactions noted in 2 or more participants who received MACRILEN.
Table 3. Frequency of Adverse Reactions Noted in Two or More MACRILEN Recipients
| N of Participants 154 | Percentage | |
|---|---|---|
| Dysgeusia | 7 | 4.5 |
| Dizziness | 6 | 3.9 |
| Headache | 6 | 3.9 |
| Fatigue | 6 | 3.9 |
| Nausea | 5 | 3.2 |
| Hunger | 5 | 3.2 |
| Diarrhea | 3 | 1.9 |
| Upper Respiratory Infection | 3 | 1.9 |
| Feeling hot | 2 | 1.3 |
| Hyperhydrosis | 2 | 1.3 |
| Nasopharyngitis | 2 | 1.3 |
| Sinus bradycardia | 2 | 1.3 |
MACRILEN Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: Overall, the risk of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races was small. Therefore, differences in side effects in other races could not be determined.
- Age: The majority of patients were younger than 65 years of age. The number of patients older than 65 years of age was small. Therefore, differences in side effects in patients older than 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The frequency of side effects noted in the clinical trials among sex, race and ager groups are shown in Table 4. There were too few patients in racial categories other than White and too few patients older than 65 years of age to allow meaningful analysis.
Table 4. Summary of Frequency of Adverse Reactions (ARs) in MACRILEN Recipients by Sex, Race and Age Category
| MACRILEN | Percentage | |
|---|---|---|
| N of MACRILEN recipients with any AR | 39/154 | 25 |
| Sex | ||
| Men | 20/90 | 22 |
| Women | 18/64 | 28 |
| Race | ||
| White | 34/132 | 28 |
| Black/African American | 0/3 | 0 |
| Asian | 2/5 | 40 |
| Other and Unknown | 3/14 | 21 |
| Age Category | ||
| 18-64 years | 38/151 | 25 |
| ≥65 years | 1/3 | 33 |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved MACRILEN based on evidence from a clinical trial (NCT02558829) in 154 participants who received MACRILEN, some were patients suspected of having growth hormone deficiency and some were healthy volunteers. The trial was conducted at 30 sites in 9 countries: 25 sites were in Europe (Austria, Germany, Spain, France, Italy, Poland, Serbia and the UK) and 5 sites were in the United States.
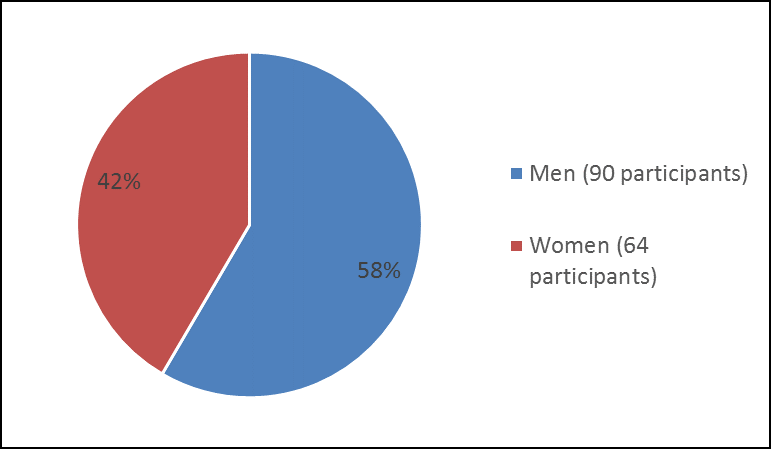
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex – Safety Population
Clinical Trial Data
Figure 2 summarizes the percentage of participants by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race – Safety Population
Clinical Trial Data
Table 1. Demographics by Race – Safety Population
| N of Participants | Percentage | |
|---|---|---|
| White | 132 | 86 |
| Black/African American | 3 | 2 |
| Asian | 5 | 3 |
| Other and Unknown | 14 | 9 |
Clinical Trial Data
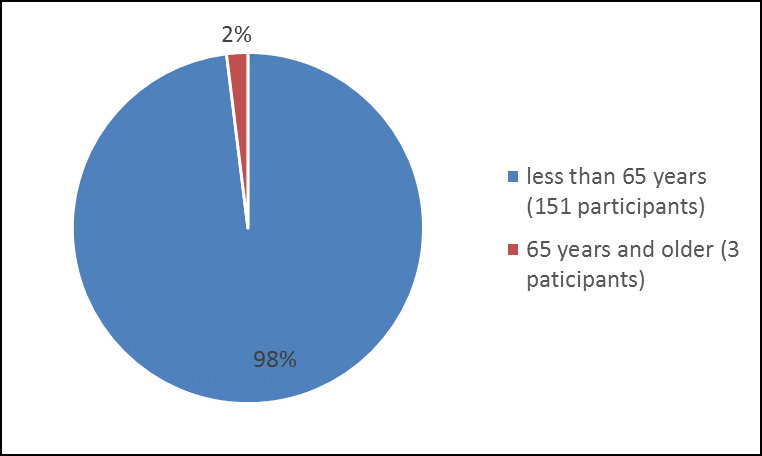
Figure 3 summarizes the percentage of participants by age group enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age – Safety Population
Clinical Trial Data
Who participated in the trials?
The safety population included 154 participants who received MACRILEN. Of these, 140 received both MACRILEN and insulin tolerance tests and were included in the efficacy analysis of the performance of the MACRILEN test.
Tables 5 and 6 summarize the demographics of participants who were included in the safety and efficacy analyses respectively.
Table 5. Demographics of Safety Population
| MACRILENN = 154 | Percentage | |
|---|---|---|
| Sex | ||
| Men | 90 | 58 |
| Women | 64 | 42 |
| Race | ||
| White | 132 | 86 |
| Black or African American | 3 | 2 |
| Asian | 5 | 3 |
| Other and Unknown | 14 | 9 |
| Age, median, (range)) | 41 (18-66) | |
| Age Category | ||
| 18 – 64 years | 151 | 98 |
| 65 years and older | 3 | 2 |
| Ethnicity | Not Reported | |
| Geographic Region | ||
| European Union | 118 | 76 |
| United States | 36 | 24 |
Clinical Trial Data
Table 6. Demographics of Participants who received Both MACRILEN and Insulin Tolerance Tests (Efficacy Population)
| Demographic Category | MACRILENModified Intent-to-Treat Population*N = 140 | Percentage |
|---|---|---|
| Sex | ||
| Men | 83 | 59 |
| Women | 57 | 41 |
| Race | ||
| White | 121 | 86 |
| Black or African American | 3 | 2 |
| Asian | 4 | 3 |
| Other and Unknown | 12 | 9 |
| Age, median, range (years) | 41 (18-66) | |
| Age Category | ||
| 18 – 64 years | 139 | 99 |
| 65 years and older | 1 | 1 |
| Geographic Region | ||
| European Union | 109 | 78 |
| United States | 31 | 22 |
*Modified Intent-to-Treat included participants who underwent both MACRILEN test and insulin tolerance test
Clinical Trial Data
How were the trials designed?
Trial participants were patients with suspected growth hormone deficiency as well as healthy volunteers. Trial participants were given MACRILEN by mouth once. Blood levels of growth hormone were measured before and up to 90 minutes after receiving MACRILEN. At a different time, the same participants were tested for growth hormone deficiency using a commonly used test (insulin tolerance test) where growth hormone levels are also measured before and after the test.
The benefit of MACRILEN in detecting hormone deficiency was assessed through comparison between the two tests.
How were the trials designed?
The clinical trial (NCT02558829) was an open-label, randomized, single-dose, two-way, cross-over comparing MACRILEN to the insulin tolerance test in the diagnosis of adult growth hormone deficiency (AGHD) in patients with high, intermediate and low likelihood of having AGHD and in a population of healthy subjects matched by age, body mass index, and estrogen status (females only). Change in growth hormone levels from baseline were measured at 30, 45, 60 and 90 minutes following ingestion of MACRILEN. The same participants also received the insulin tolerance test and changes in growth hormone levels from baseline were measured. Agreement between the two test was the primary efficacy outcome of the trial.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.