Testimony | In Person
Event Title
Examining FDA’s Medical Device User Fee Program
March 27, 2017
- Testimony of
-
Testimony of Jeffrey Shuren, M.D., J.D.
Director, Center for Devices and Radiological Health
Before the
United States House of Representatives
Committee on Energy and Commerce Subcommittee on Health
March 28, 2017
INTRODUCTION
Chairman Walden, Ranking Member Pallone, Chairman Burgess, Ranking Member Green, and members of the committee:
Thank you for having me here today. I’m Jeff Shuren, Director of the Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA). I’m pleased to be here today to discuss reauthorization of the Medical Device User Fee Amendments, or MDUFA IV.
The MDUFA reauthorization proposal described below was submitted to Congress in January under the previous Administration, and reflects a different approach to the Federal Budget. The Blueprint Budget supports many of the goals of the reauthorization proposal but proposes a different way of financing these goals. The Administration looks forward to working with Congress, with industry input, to develop a reauthorization proposal that speeds the development and approval of vital medical devices that are safe and effective.
MDUFA
Enacted by Congress in 2002, MDUFA is a user fee program through which medical device companies pay fees to FDA when they submit a request for marketing authorization or register their establishments with FDA. The program includes commitments between the U.S. medical device industry and FDA to improve the predictability, transparency, and consistency of regulatory processes, which are intended to reduce the time for FDA to make a decision about whether to authorize marketing of a device.
MDUFA has been reauthorized every five years since Congress created the program. As the program has evolved, FDA and industry have successfully negotiated agreements to improve patient access to medical devices and streamline regulatory processes.
During the 2012 MDUFA III testimony, many of you may recall that the program was in a much different place1 :
- In FY 2009, it took an average of 427 days to reach a decision on a premarket approval application (PMA), the submission type required for the highest-risk devices.
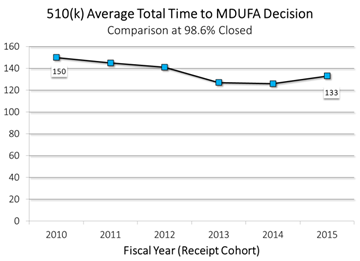
- In FY 2010, it took an average of 150 days to reach a decision on a premarket notification submission (also known as a 510(k)), the submission type required for low to moderate-risk devices.
Thanks to the investment provided by industry, and direction provided by Congress, we have made substantial progress toward reducing decision times. As of 2015:
- It took an average of just 276 days to reach a decision on a PMA, a 35 percent decrease in six years; and
- It took an average of just 133 days to reach a decision on a 510(k), an 11 percent decrease in five years.
Further, we went beyond our MDUFA III commitments to reduce the median time to approve an Investigational Device Exemption (IDE) study to just 30 days in FY 2015, down from 442 days in FY 2011—a 93 percent decrease in four years. This improvement has allowed companies to begin their clinical trials earlier so they can begin collecting data to support a decision on their submission requesting marketing authorization. In addition, we reduced the average time to reach a decision on a De Novo classification request, the submission type typically used by novel low or moderate-risk devices, to 259 days in FY 2014, down from 770 days in FY 2009—a 66 percent decrease in five years.
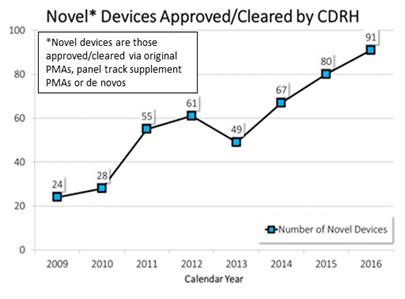
Changes we have made at CDRH to our culture, policies, and processes—in addition to user fee funding and changes to federal law—have resulted in an improved medical device pipeline and innovative technologies being introduced in the U.S. earlier than in the past. For example, since 2009, the number of innovative devices we have approved has almost quadrupled. In 2016, we approved 91 innovative devices—the highest of any year since the user fee program began in 2003. In 2015, we approved the second highest number of innovative devices.
An example of an innovative technology that FDA approved first in the world is the “artificial pancreas,” something many members of this Committee supported. Working interactively with the device manufacturer from the earliest stages of development to assist in making this technology available as quickly as possible, FDA approved the first device in the world that is intended to automatically monitor glucose levels around the clock and automatically provide appropriate insulin doses.
While we have made progress in many areas, we also recognize that more work remains and there are additional opportunities for improvements. We look forward to working with industry and Congress to ensure there are sufficient user fees resources as we strive to make these improvements. MDUFA IV agreement includes a new quality management program that will enhance consistency and predictability in premarket review processes.
MDUFA IV agreement would also allow FDA to move forward in some critical and strategic areas such as strengthening our partnerships with patients2 . Strengthening patient input will allow us to promote more patient-centric clinical trials, advance benefit-risk assessments that are informed by patient perspectives, and foster earlier access to new devices.
Another critical area supported by the MDUFA IV agreement is the development of the National Evaluation System for health Technology, or NEST3 . The NEST is system owned and operated by multiple stakeholders that will use real-world data collected as part of routine clinical care. A robust NEST will enable manufacturers to harness real-world evidence that could enable them to drive down the time and cost of bringing a new device to market, expand the indications for already approved devices, and meet postmarket reporting requirements. The NEST will also enable faster identification of safety issues, reducing harm to patients and liability for companies.
The MDUFA IV agreement, which was supported by a broad array of stakeholders during the public review of the draft agreement, will expedite the availability of innovative new products, and its enhancements will continue to increase the efficiency of FDA’s programs. Improvements in total time to decision, transparency, consistency, and predictability will benefit industry, healthcare providers, and, most importantly, patients.
CONCLUSION
The Medical Device User Fee Program has allowed FDA to speed the application review process without compromising the Agency’s high standards. MDUFA offers a strong example of what can be achieved when FDA, industry, and other stakeholders work together towards the same goal. The user fees provide a critical way to ensure that FDA has the resources needed to conduct reviews in a timely fashion. While we have made demonstrable progress in partnering to bring medical devices to market in as timely a manner as possible, we know that more work remains to be done to further enhance and optimize our processes. The reauthorization MDUFA will allow FDA to build upon the demonstrated success of this program, and in so doing, further benefit patients and affirm our nation’s standing as a global leader in biomedical innovation.
Appendix A
U.S. Food and Drug Administration
Center for Devices and Radiological Health:
Progress in Achieving Our Vision of Patients First
In the early part of this decade, industry argued that FDA regulation hindered innovation and contributed to the growing number of device companies seeking marketing authorization for their devices abroad before introducing them in the United States, and the increasing gap between when a device is approved in another country and when it is approved in the US. This reality, its adverse impact on patients, plus CDRH’s own awareness of our declining performance over almost a decade, led CDRH to implement new programmatic changes. These changes, along with increased user fee funding and changes in Federal law have helped us strengthen our performance and better address the rapidly-evolving field of medical device innovation. To guide us in our mission to improve the health and quality of life of patients, in 2012 we adopted a new vision4 to reflect this change in mindset, that: Patients in the US have access to high-quality, safe and effective medical devices of public health importance first in the world.
Doing Business Better
Since late 2009, CDRH has continuously improved the way we do business through a series of culture, policy and process changes. This can be seen through our commitment to providing excellent customer service, new patient-centered paradigms, and our strong performance across a range of objective measures, including the time it takes to review several types of medical device submissions. These improvements are reflected by the nearly four-fold increase in the annual number of novel medical device approvals.
Fast Facts CDRH oversees approximately 175,000 medical devices on the US market, more than 18,000 medical device manufacturers, and more than 25,000 medical device facilities worldwide. Each year we receive some 22,000 premarket submissions (includes supplements and amendments) and more than 1.4 million reports on medical device adverse events and malfunctions.
Time
Time, with its cost implications, plays a critical role in an innovator’s decision as to whether and when to bring a new technology to the US. What good is a new technology if patients do not have timely access to it? How helpful is a new technology that doesn’t benefit patients or poses unacceptable risks? By reducing the time of every regulatory stage of the total product life cycle, including the review of medical device submissions, while still assuring robust but appropriate (least burdensome) evidence generation and high-quality decision making, we help patients get access to safe and effective medical technologies and foster innovation. After steadily worsening performance from 2002 to 2010 on a variety of measures, including premarket review times, CDRH has reduced the decision time on all key premarket submission types.
PMA While premarket approval applications (PMAs) only account for approximately one percent of all premarket medical device submissions, they represent medical devices with the highest risk to patients (Class III devices) and, therefore, require more data and a more rigorous review by CDRH. In 2009, it took an average of 427 total days to reach a decision on a PMA. By 2015, we had reduced the total decision time by 35 percent.
510(k) Named after its section number in federal law, this category represents the bulk of premarket submissions for medical devices. Manufacturers submit 510(k)s to CDRH for devices with low to moderate risk to patients (Class II), and our review standard is based on substantial equivalence (whether a device is at least as safe and effective as a device already on the market). In 2010, it took an average of 150 total days to reach a decision on a 510(k). By 2015, we had reduced the total decision time by 11 percent.
De Novo De novo classification is a pathway that enables manufacturers of certain low to moderate risk novel devices for which there are no similar marketed devices to come to market, instead of having to submit a PMA. In 2009, it took an average of 770 total days to reach a de novo decision. By 2014, we had reduced the total decision time by 66 percent.
IDE Manufacturers submit Investigational Device Exemptions (IDEs) for certain devices they want to study via a clinical trial. CDRH reviews an IDE submission before a manufacturer can begin to collect clinical data that may be necessary for future approval. CDRH slashed median review times for IDE full approvals by more than a year between 2011 and 2015.
Doing Business Different
Since 2009, CDRH has been evaluating all of our programs to address concerns from patients, industry, health care providers, our own staff, and other customers about issues including review times, backlogs, and our expertise in increasingly complex technology. We have sought to address these concerns by changing our culture to put patients first and recognizing that advancing innovation and assuring patient safety are not mutually exclusive, revising or eliminating old policies, and developing new policies and approaches with an eye on meeting measurable objectives. Increased medical device user fees have supported these efforts so that we are better positioned to respond to the needs of patients.
Clinical Trials
In addition to dramatically improved performance5 in reviewing IDEs, CDRH has encouraged the use of innovative methodologies and study designs in clinical trials. We recognize that manufacturers need CDRH input early and often so that the ultimate device review process moves as quickly and smoothly as possible. In 2013, CDRH issued final guidance for manufacturers on early feasibility studies to encourage conducting these studies in the US. Innovators tend to market their technologies sooner in countries where they elect to conduct their early clinical studies. Since 2013, the number of early feasibility studies approved has more than doubled—from 17 in FY 2013 to 40 in FY 2016.
CDRH encourages the use of innovative clinical trial designs and statistical methods such as adaptive clinical trials6 and Bayesian statistics7 because, where appropriate to use, they can reduce the time and cost of a clinical study. In recent years, many devices have come to market based on the results of clinical trials using adaptive trial designs. For the period from 2007 to May of 2013, CDRH received 201 submissions that were adaptive.
CDRH continues to develop computational models that can, in some instances, supplement or replace data from clinical investigations, such as the Virtual Family (VF)8—a set of highly detailed, anatomically correct, computational whole-body models, designed to mimic humans of both sexes at various stages of growth. Since 2007, more than 160 submissions have included Virtual Family research.
Flexible, Risk-Based Regulatory Approaches
CDRH continues to adapt our oversight policies to emerging new technologies. In a manner consistent with our statutory mission, we now approach a medical technology by first asking whether active CDRH oversight will be value-added. If not, we take a less active regulatory approach. If it would, we focus on assuring timely patient access to technologies that will benefit patients by considering the device’s innovation cycles and evidence generation needs.
For example, widespread adoption and use of digital health technologies is creating new and innovative ways to improve health and health care delivery. In one of the biggest de-regulatory actions for CDRH in decades, to foster greater innovation in the digital health space while promoting public health, we have exercised our enforcement discretion to cease subjecting certain lower-risk medical devices (such as apps for patient care management and medication reminders) to medical device requirements.
Additionally, balancing data needs between what’s collected before the device comes on the market (premarket) and what’s collected after it is on the market (postmarket) reflects our approach to best assure timely patient access to safe and effective devices.
In 2015, CDRH completed a retrospective review9 of the benefit-risk profile of all types of high-risk devices to determine if we could reduce premarket data collection requirements for at least some devices. As a result, for 30 percent of high-risk medical devices, CDRH determined, based on the current body of evidence and experience, we could consider some devices candidates for down-classification, eliminate some data requirements or shift some premarket data requirements to the postmarket setting. In 2016, CDRH reached out to stakeholders for input on the results of the retrospective reviews, in order to determine next steps.
Patient-Centered Benefit-Risk
For the past 5 years, CDRH has encouraged the use of a more flexible, patient-centric, and transparent benefit-risk framework to evaluate medical devices, starting with a 2012 guidance on the factors to consider when making benefit-risk determinations in support of device premarket approval decisions, which includes patient perspectives on potential benefits and risks. We are focusing more on what matters to patients.
In 2016 and 2017, CDRH expanded this approach by revising the 2012 guidance to include additional patient-centric factors10 , and issuing two additional benefit-risk guidance documents: one which outlines the principal factors CDRH considers when making benefit-risk determinations during the premarket review process for IDEs11, and one which outlines factors to consider when determining whether and what postmarket actions12 we may take to address a problem, such as a recall, based on the benefits and risks of that action to patients.
Patients as Partners
CDRH had traditionally determined whether the benefits of a device outweighed its risks based on the trade-offs we thought were acceptable. However, patients who live with a disease or condition often have their own perspectives on what benefits and risks related to medical devices they are willing to accept. CDRH collaborates with patient scientists and other experts outside the FDA to help us advance the scientific field of assessing patient preferences and incorporate the patient perspective into our benefit-risk assessments and decision-making.
For example, in 2014, CDRH funded a collaborative study on patient preferences that led to changes in our review paradigm for obesity devices, and used the results to inform our decision to approve the first medical device for treating obesity since 2007. Better understanding of patient preferences can also help rejuvenate development pipelines; since then, CDRH has approved or granted marketing applications for five more medical devices that address obesity or weight loss.
In 2016, CDRH issued a final guidance that outlined patient preference information (PPI)13 that CDRH may use in decision making. Since then manufacturers have begun to submit—and we have approved—IDEs with patient preference studies.
CDRH’s efforts to incorporate the voice of patients in our decision making also are reflected in medical device clinical studies, which have been increasingly assessing what matters most to patients. Between 2009 and 2014, the number of premarket submissions that included clinical studies with patient reported outcomes (PROs) increased by more than 500 percent and half of IDE pivotal clinical studies now include PROs.
In 2015, CDRH established the first FDA advisory committee focused on the interests and needs of patients, and recruited potential new members in 2016. The Patient Engagement Advisory Committee14 will hold its first meeting in 2017.
National Evaluation System for health Technology (NEST)
Despite rigorous premarket evaluation, we cannot fully understand how well a medical device works until it is used day-to-day by patients, caregivers, and clinicians. Premarket clinical trials provide critically important information but we don’t understand the long-term benefit-risk profile until it is used in routine clinical practice. Currently our nation is limited in its ability to make widespread use of real-world evidence (RWE) to best inform all members of the medical device ecosystem.
CDRH intends for NEST to increase the quality and use of real-world data (RWD) collected as part of routine clinical care, which should also help reduce the time and cost of evidence generation. Ongoing implementation of the Unique Device Identification (UDI) system also will enable NEST to perform enhanced analyses of devices on the market, providing a clear and standard way to identify devices in electronic medical records.
CDRH is already relying on RWE to approve new devices, expand the indications for already marketed devices, and reduce the time and cost for device makers to meet their postmarket study requirements. In 2016, CDRH documented access to more than 28 million electronic patient records (from national and international clinical registries, claims data, and electronic health records) that included device identification and awarded $3 million to the Medical Device Innovation Consortium to establish the NEST Coordinating Center.
Streamlining the Pathway from FDA Approval to Payer Coverage
Timely access to innovative medical technologies has been identified as a significant issue in the delivery of high quality health care. Manufacturers of innovative medical products have said that after undergoing the FDA approval process the availability of their products to consumers is often slow because, in order to obtain coverage and payment from third-party payers, the manufacturers must go through a second review process by such payers. Therefore, CDRH established the Payer Communication Task Force (PCTF) to facilitate communication between device manufacturers and payers to shorten the time between FDA approval or clearance and coverage decisions. By communicating earlier, manufacturers may design their pivotal clinical trials to produce both the data required for regulatory approval or clearance, and positive coverage determinations.
To support these efforts, CDRH and the Centers for Medicare & Medicaid Services (CMS) began to pilot an approach in 2011 called Parallel Review that would give eligible device makers the voluntary option for CMS to start their national coverage determination process while the device is under review by CDRH. This process serves the public interest by reducing the time between FDA marketing approval or clearance decisions and CMS national coverage determinations. In 2016, CDRH and CMS established Parallel Review as a permanent program. Last year, CDRH also established an additional opportunity for device manufacturers to invite CMS, private payers, or health technology assessment groups (HTAs) to join FDA pre-submission meetings to provide early feedback on clinical trial design.
EVIDENCE OF IMPACT
Our investments are starting to pay off. For example, in 2016, CDRH approved 91 novel medical devices—the highest number since the advent of the user fee program in 2003. This followed the second highest number from 2015, and continued a 7-year trend that has resulted in a marked increase in the annual number of novel device approvals since 2009. These novel technologies, which can help improve the quality of life of patients, especially those that require day-to-day maintenance and ongoing attention, are yielding promising results. In addition, several of these devices are reaching US patients much earlier than they would have in previous years.
“Artificial Pancreas”
Approximately five percent of diabetics have Type 1 diabetes, also known as juvenile-onset diabetes. People with type 1 diabetes have to constantly monitor their glucose levels throughout the day and have insulin therapy through injection with a syringe, an insulin pen, or an insulin pump, to avoid becoming hyperglycemic (high glucose levels). Working interactively with the sponsor from the earliest stages of development to assist in making this technology available as quickly as possible while assuring it is safe and effective, CDRH, in 2016, approved the first automated insulin delivery (AID) device in the world that is intended to automatically monitor glucose (sugar) and provide appropriate basal insulin doses—what some have called a first-generation “artificial pancreas15.”
Transcatheter Aortic Valve Replacement (TAVR) Therapy
About 80,000 surgical aortic valve replacements (SAVR) are performed in the US annually. One-third of these patients are at intermediate surgical risk for death or complications. An aortic valve replacement that can be inserted through the blood vessels or, in some cases, through the tip of the heart by a catheter, rather than through open surgery, could avoid the risks of surgery and provide an alternative effective treatment to patients who are in the “intermediate surgical risk” category.
In 2011, CDRH approved the first TAVR device in the US for patients who are not surgical candidates for SAVR, more than four years after the device entered the European Union (EU) market. When, in 2016, CDRH approved the expanded indication16 for use for a TAVR device in patients at intermediate surgical risk for death or complications, the positive impact of CDRH initiatives was evident. The gap between EU and US approval for the expanded indication for use was reduced from over four years to only 18 days. US Medicare coverage is also a factor in patients’ access to devices. For TAVR devices, access to real-world evidence—what NEST hopes to expand—proved to be a valuable asset. The US Medicare program immediately covered TAVR devices due to the ongoing collection of real-world evidence on these devices in a national registry—there was no delay between US approval and access to this technology. As a result, more than 25,000 additional patients each year are now eligible for this life-saving procedure.
Diagnostics for National Emergencies
Accurate detection and diagnostics are critical to addressing national public health threats. For example, in 2016, CDRH authorized the use of fourteen diagnostic tests for Zika17 virus under our Emergency Use Authorization (EUA) authority—twelve tests to diagnose active infection and two tests to assess whether individuals who may have recently been exposed to Zika were actually infected. This rapid action provided timely patient access to Zika tests before the summer of 2016, when officials detected the virus in the U.S. Since 2009, CDRH has granted 50 EUAs, reauthorized 19 EUAs, and granted 30 amendments for tests to help meet the country’s needs during a national public health emergency, such as outbreaks from Zika, Ebola, and H1N1.
Appendix B
Center for Devices and Radiological Health (CDRH)
2016-2017 Strategic Priorities - 2016 Accomplishments
Establish a National Evaluation System for Medical Devices
To successfully harness real-world evidence (”evidence from clinical experience”) in an efficient manner, the U.S. must develop the necessary infrastructure – a National Evaluation System for health Technology (NEST).
Goal: Increase Access to Real-World Evidence to Support Regulatory Decision Making
| 2016 Target | Results |
|---|---|
| 25 Million By December 31, 2016, gain access to 25 million electronic patient records (from national and international clinical registries, claims data, and EHRs) with device identification. | 28.6 Million Gained access to more than 28 million electronic patient records (from national and international clinical registries, claims data, and EHRs) with device identification using a variety of mechanisms, such as cooperative agreements and access through regulatory process. |
Goal: Increase the Use of Real-World Evidence to Support Regulatory Decision Making
|
2016 Target |
Results |
|---|---|
|
40% increase. By December 31, 2016, increase by 40 percent the number of premarket and postmarket regulatory decisions that leverage real-world evidence. (compared to FY2015 baseline) |
85% increase. The number of premarket and postmarket regulatory decisions that used real-world evidence increased by 85 percent in 2016. (compared to FY2015 baseline) |
Supporting Actions
In 2016, CDRH took a number of actions to achieve the goals and targets established for this priority.:
Establish the National Evaluation System for health Technology (NEST)
In Progress: A multi-stakeholder Planning Board and the Medical Device Registry Task Force issued a series of reports that outlined an organizational structure and infrastructure for the NEST Coordinating Center (February 201518, April 201619, September 201620, and August 201521). In 2016, FDA awarded $3 million to the Medical Device Innovation Consortium (MDIC) to establish the Coordinating Center, and $1 million to other organizations to continue projects that generate real-world evidence on device performance.
Develop a framework for the incorporation of real-world evidence into regulatory decision making.
In Progress:Issued draft guidance22 to describe how real-world evidence may be used to support pre- and postmarket regulatory decisions. Final guidance is planned for 2017.
Partner with Patients
We believe that if CDRH is to successfully achieve a mission and vision in the service of patients, we must interact with patients as partners and work together to advance the development and evaluation of innovative devices, and monitor the performance of marketed devices.
Goal: Promote a Culture of Meaningful Patient Engagement by Facilitating CDRH Interaction with Patients
| 2016 Target | Results |
|---|---|
| 10 Organizations By December 31, 2016, establish one or more new mechanisms for CDRH employees to obtain patient input on key pre- and postmarket issues facing CDRH and foster participation of 10 patient groups. | 34 Organizations CDRH staff participated in 21 patient interaction opportunities, involving 34 patient organizations. |
| 50% By December 31, 2016, 50 percent of CDRH employees will interact with patients as part of their job duties. | 68% More than 68 percent of CDRH interacted with patients in 2016. When asked, 99 percent of staff who interacted with patients described their interaction as meaningful and 89 percent as relevant to their jobs. |
Goal: Increase Use and Transparency of Patient Input as Evidence in Our Decision Making
| 2016 Target | Results |
|---|---|
| 50% By September 30, 2016, 50 percent of PMA, de novo and HDE decisions will include a public summary of available and relevant patient perspective data considered. | 65% In FY 2016, 65 percent of PMA, de novo, and HDE decisions included a public summary of available patient perspective data. |
| Increase. By September 30, 2017*, increase the number of patient perspective studies (e.g., evaluating patient reported outcomes (PRO) or patient preference information (PPI)) used in support of premarket and postmarket regulatory decisions. (compared to FY 2015 baseline) *2017 Target |
65% Increase. PRO and 4Ý PPI Increased by 65 percent the number of approved IDEs (pivotal studies only) with patient reported outcomes (PRO). Increased to four (from none) the number of patient perspective studies conducted by sponsors in support of pre- and postmarket regulatory decisions. |
Supporting Actions
In 2016, CDRH took a number of actions to achieve the goals and targets established for this priority:
Patient Engagement Advisory Committee Convene the Patient Engagement Advisory Committee to discuss high priority topics regarding patient input in the total product lifecycle.
In Progress: CDRH chartered and began to recruit members for FDA’s new Patient Engagement Advisory Committee (PEAC). PEAC members will be selected and announced in 2017.
Education and Training Develop education and training for CDRH staff and industry on the development and use of the science of measuring and communicating patient input throughout the total product lifecycle.
In Progress: CDRH trained more than 80 staff members on patient reported outcomes (PRO) and patient preference information (PPI), to advance staff understanding and CDRH review capacity in these areas.
Promote a Culture of Quality and Organizational Excellence
A manufacturer’s ability to design and make high-quality, safe and effective devices and CDRH’s ability to provide the necessary oversight to assure devices on the market are high-quality, safe and effective will increase as manufacturers and CDRH embrace a culture of quality and excellence throughout our respective organizations.
Goal: Strengthen FDA’s Culture of Quality within the Center for Devices and Radiological Health
| 2016 Target | Results |
|---|---|
| 10% Increase. By September 30, 2016, increase by 10 percent the number of CDRH staff with quality and process improvement credentials to improve organizational excellence. (compared to FY 2015 baseline) | 300% Increase. In FY 2016, CDRH tripled the number of staff with quality credentials by providing on-site quality training and certification examinations. |
Goal: Strengthen Product and Manufacturing Quality within the Medical Device Ecosystem
| 2016 Target | Results |
|---|---|
| By September 30, 2016, develop metrics, successful industry practices, standards, and tools that manufacturers can use to evaluate product and manufacturing quality beyond compliance with regulatory requirements. | Partnered with MDIC to develop metrics and best practices to assess quality system performance, and analytical tools to assess device quality by hospital value analysis committees. |
| By December 31, 2016, pilot voluntary use of product and manufacturing quality metrics and evaluation tools. | Partnered with MDIC and Capability Maturity Model Integration (CMMI) Institute on a proof-of-concept and pilot with three device manufacturers, to evaluate use of the CMMI appraisal process as a foundation for a future third party program. |
Supporting Actions
In 2016, CDRH took a number of actions to achieve the goals and targets established for this priority:
Quality Management Framework Resources permitting, continue to implement the CDRH Quality Management Framework.
In Progress: CDRH completed development of its document control system (DCS). DCS will ensure that current and approved quality program and key processes documentation—standard operating procedures, work instructions, forms, templates and process maps—is available to staff.
Education and Training Develop education and training for CDRH staff to facilitate adoption of practices characteristic of a culture of quality and organizational excellence.
In Progress: CDRH became an American Society for Quality (ASQ) enterprise member—enabling every employee at FDA to take advantage of ASQ’s vast collection of learning resources. CDRH also offered on-site quality training to 150 staff. More than 90 percent of those who participated in the training earned ASQ quality certifications (Certified Quality Auditor and Certified Quality Improvement Associate).
Case for Quality As part of the Case for Quality, collaborate with members of the medical device ecosystem to identify, develop, and pilot metrics, successful practices, standards, and evaluation tools that will be specific to the medical device industry and focus on assuring product and manufacturing quality.
In Progress: In partnership with MDIC, CDRH collected input from stakeholders through six Case for Quality Forums; developed metrics and best practices designed to assess quality system performance using pre-production, production and post-production data; and led development of a product quality dashboard to assist hospital value analysis committees in identifying high quality devices.
Voluntary Program Identify external partnerships and mechanisms to support a sustainable, voluntary third party program that will utilize quality metrics, practices, standards, and evaluation tools to assess and promote medical device product and manufacturing quality within industry beyond compliance with regulatory requirements.
In Progress: Continuing partnership with MDIC, CMMI Institute and other stakeholders, to expand application of maturity appraisal process; with the goal of developing the framework for a voluntary program in 2017.
Footnotes
1. See Appendix A: “U.S. Food and Drug Administration, Center for Devices and Radiological Health:
Progress in Achieving Our Vision of Patients First.”
2. See Appendix B: “Center for Devices and Radiological Health (CDRH): 2016-2017 Strategic Priorities – 2016 Accomplishments.”
3. See Appendix B: “Center for Devices and Radiological Health (CDRH): 2016-2017 Strategic Priorities – 2016 Accomplishments.”
10. Guidance Document: Factors to Consider When Making Benefit-Risk Determinations in Medical Device Premarket Approval and DeNova Classification (Aug. 24, 2016)
11. Guidance Document: Factors to Consider When Making Benefit-Risk Determinations in Medical Device Investigational Device Exemptions
12. Guidance Document: Factors to Consider When Making Benefit-Risk Determinations in Medical Device Product Availability, Compliance, and Enforcement Decisions
13. Guidance Document: Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling
16. Press Release: FDA approves expanded indication for two transcatheter heart valves for patients at intermediate risk for death or complications associated with open-heart surgery – Aug. 2016
19. The National Evaluation System for health Technology: Priorities for Effective Early Implementation; Planning Board Report