Regulatory Pharmaceutical Fellowship Program
The U.S. Food and Drug Administration (FDA) partners with universities and colleges of pharmacy in the United States to offer FDA-affiliated fellowship programs to Doctor of Pharmacy graduates in several specialties. The purpose of the Regulatory Pharmaceutical Fellowship program is to train selected candidates in one of six tracks focused on drug information, medication safety, regulatory advertising & promotion, regulatory affairs and policy, biopharmaceutical manufacturing, or regulatory science. The program provides participants with the unique opportunity to learn from mentors in their chosen specialty track across three diverse settings in government, academia, and industry. Graduates of the fellowship program are qualified to pursue careers in any of the three practice settings.
Recruitment is open for 2025-2027 positions on a rolling basis. See program specific information below for important dates and links to more information.
Biopharmaceutical Manufacturing
Two-Year Biopharmaceutical Manufacturing Fellowship provided by Albany College of Pharmacy and Health Sciences
Fellowship dates: TBD, typical timeline shown below.
- Four months with the Stack Family Center for Biopharmaceutical Education and Training (CBET) at Albany College of Pharmacy and Health Sciences in Albany, NY
- Twelve months at Curia Global in global contract research, development and manufacturing at one or more locations
- Eight months at FDA in the Office of Pharmaceutical Manufacturing Assessment in the Office of Pharmaceutical Quality in Silver Spring, MD
Visit Albany College of Pharmacy and Health Sciences Fellowship website for eligibility criteria and key application dates. Questions? Contact Bernard.Tyrrell@acphs.edu.
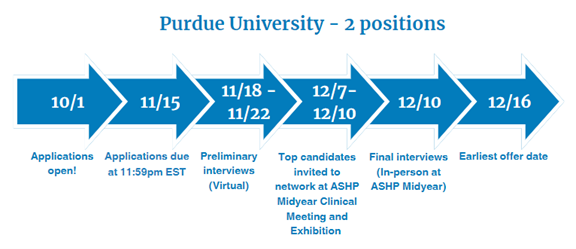
Drug Advertising and Promotion
Two-Year Regulatory Advertising and Promotion Fellowship provided by Purdue University
Fellowship dates: July 1, 2025 – June 30, 2027
- Six months at Purdue University in Indianapolis, IN
- Nine months at Johnson & Johnson in Titusville, NJ
- Nine months at FDA in the Office of Prescription Drug Promotion in the Center for Drug Evaluation and Research in Silver Spring, MD (currently a remote work environment)
For more information, please see this brochure.
Visit Purdue University's Drug Advertising and Promotion Fellowship website for eligibility criteria and key application dates. Questions? Contact AdPromoFellowship@purdue.edu
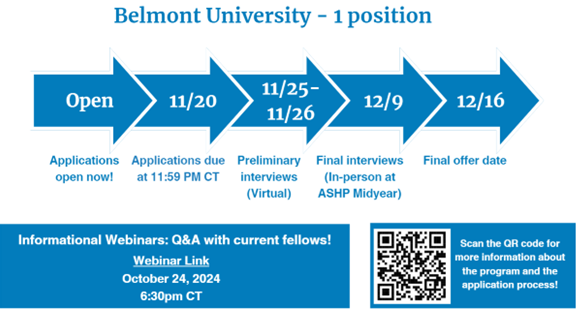
Drug Information - 1 position
Two-Year Drug Information Fellowship provided by Belmont University
Fellowship dates: June 2, 2025 – May 31, 2027
- Seven months will be spent at Belmont University with practice site responsibilities in the HealthTrust Drug Information Service in Nashville, TN
- Six months will be spent at Belmont University in the Christy Houston Foundation Drug Information Center in Nashville, TN
- Five months will be spent at HealthTrust GPO Operations in Nashville, TN
- Six months will be spent at FDA in the Division of Drug Information in the Center for Drug Evaluation and Research in Silver Spring, MD
For more information, please see this brochure.
Visit Belmont University's Drug Information Fellowship website for eligibility criteria and key application dates. Questions? Contact druginfo@belmont.edu.
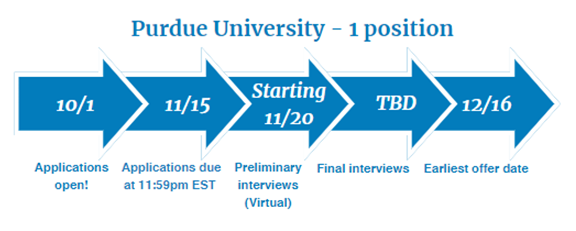
Drug Information - 2 positions
Two-Year Drug Information Fellowship provided by Purdue University
Fellowship dates: July 1, 2025 – June 30, 2027.
- Six months will be spent at Purdue University in Indianapolis, IN
- Twelve months will be spent at Eli Lilly & Company in Global Medical Information in Indianapolis, IN or at Janssen Scientific Affairs, LLC in Medical Information in Horsham, PA
- Six months will be spent at FDA in the Division of Drug Information in the Center for Drug Evaluation and Research in Silver Spring, MD
For more information, please see this brochure.
Visit Purdue University's Drug Information Fellowship website for eligibility criteria and key application dates. Questions? Contact DrugInfoFellowship@purdue.edu
Medication Safety - TBA
Two-Year Pharmacovigilance Fellowship provided by Albany College of Pharmacy and Health Sciences
Fellowship dates: TBD, typical timeline shown below.
- Six months will be spent at Albany College of Pharmacy and Health Sciences in Albany, NY
- Nine months will be spent remotely at BeiGene in Global Patient Safety
- Nine months will be spent at FDA with Regulatory Science Staff in the Office of Surveillance and Epidemiology in Silver Spring, MD
Visit Albany College of Pharmacy and Health Sciences Fellowship Opportunities for eligibility criteria and key application dates. Questions? patrick.meek@acphs.edu
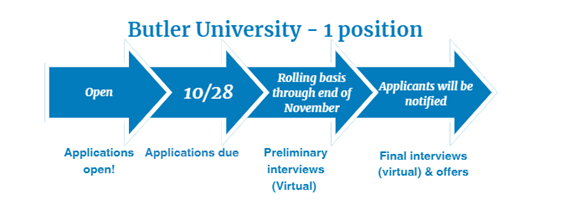
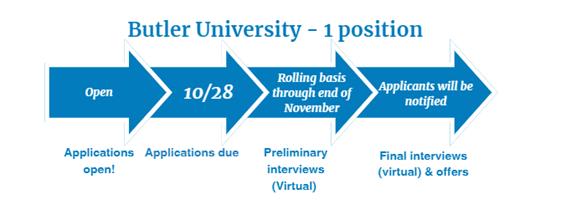
Medication Safety - 1 position
Two-Year Medication Error Pharmacovigilance and Risk Management Fellowship provided by Butler University
Fellowship dates: July 1, 2025 – June 30, 2027
- Four months will be spent at Butler University in Indianapolis, IN
- Twelve months will be spent at Regeneron Pharmaceuticals in Regulatory Affairs and Global Patient Safety in Tarrytown, NY
- Eight months will be spent at FDA in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
For more information, please see this brochure.
Visit Butler University's Pharmacy Fellowship Programs Website for eligibility criteria and key application dates. Questions? Contact rxfellowships@butler.edu
Medication Safety - 1 position
Two-Year Global Patient Safety and Pharmacovigilance Fellowship provided by Butler University
Fellowship dates: July 1, 2025 – June 30, 2027
- Four months will be spent at Butler University in Indianapolis, IN
- Twelve months will be spent at Eli Lilly in Global Patient Safety in Indianapolis, IN
- Eight months will be spent at FDA in the Division of Medication Error Prevention and Analysis in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
For more information, please see this brochure.
Visit Butler University's Pharmacy Fellowship Programs Website for eligibility criteria and key application dates. Questions? Contact rxfellowships@butler.edu
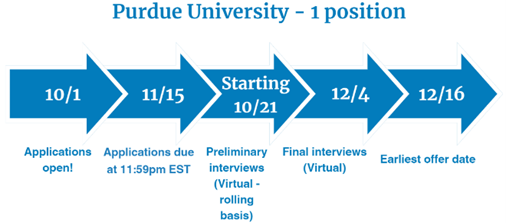
Medication Safety - 1 position
Two-Year Medication Safety Fellowship provided by Purdue University
Fellowship dates: July 1, 2025 – June 30, 2027
- Four months will be spent at Purdue University in Indianapolis, IN
- Twelve months will be spent at AbbVie in Pharmacovigilance and Patient Safety in North Chicago, IL
- Eight months will be spent at FDA in the Division of Risk Management in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
For more information, please see this brochure.
Visit Purdue University's Medication Safety Fellowship for eligibility criteria and key application dates. Questions? Contact MedSafetyFellowship@purdue.edu
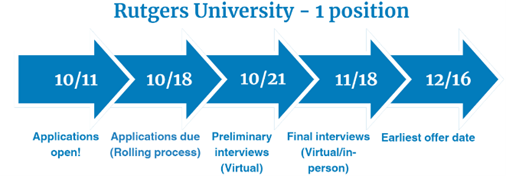
Medication Safety - 1 position
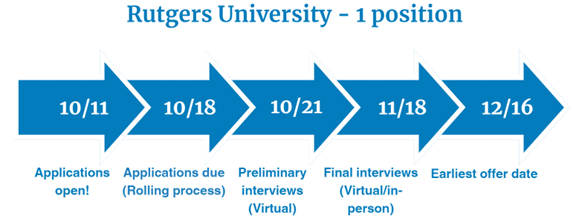
Two-Year Risk Management Fellowship provided by Rutgers University
Fellowship dates: July 1, 2025 – June 30, 2027
- A longitudinal academic experience provided by Rutgers University in Piscataway, NJ with travel expected to the campus at least once monthly throughout the program
- Twelve months at Pfizer in the Risk Management Center of Excellence in Collegeville, PA
- Twelve months at FDA the Division of Risk Mitigation Assessment and Medication Error Surveillance, in the Office of Medication Error Prevention and Risk Management in the Office of Surveillance and Epidemiology in Silver Spring, MD
For more information, please see this brochure.
Visit Rutgers Health Institute for Pharmaceutical Industry Fellowships for eligibility criteria and key application dates. Questions? Contact Carolyn.Seyss@pharmacy.rutgers.edu
Regulatory Policy and Program Management - not recruiting
Two-Year Regulatory Policy and Program Management Fellowship provided by Howard University
Fellowship dates: N/A
- A longitudinal academic experience provided by Howard University in Washington, DC with travel expected to campus for predetermined instruction and development focused on the drug development process and its requisite policy
- Eighteen months at Genentech in a rotational immersion in regulatory policy and program management in Washington, DC (travel expected as needed to the San Francisco Headquarters)
- Six months at FDA in the Office of Medical Policy in Silver Spring, MD
Visit Howard University College of Pharmacy Fellowship Program for eligibility criteria and key application dates. Questions? Contact Earl.Ettienne@Howard.edu
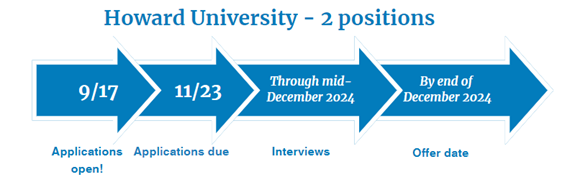
Global Regulatory Affairs and Policy - 2 positions
Two-Year Regulatory Affairs and Policy Fellowship provided by Howard University
Fellowship dates: July 1, 2025 – June 30, 2027
- Six months academic experience completing coursework and training through Howard University College of Pharmacy and School of Law
- Eighteen months at GlaxoSmithKline in a rotational immersion in regulatory advertising and promotion, strategy, and policy and intelligence. The first six months of the GSK rotation will run concurrently with the Howard rotation
- Six months at FDA in the Office of Medical Policy in Silver Spring, MD focused in either patient labeling or regulatory policy
Visit Howard University College of Pharmacy Fellowship Program for eligibility criteria and key application dates. Questions? Contact Earl.Ettienne@Howard.edu
Regulatory Science
Two-Year Regulatory Science Fellowship provided by Rutgers University
Fellowship dates: July 1, 2025 – June 30, 2027
- Eight months will be spent in a clinical rotation experience, provided by Rutgers University Ernest Mario School of Pharmacy in Piscataway, NJ at an associated clinical practice site
- Eight months will be spent at Sanofi in Regulatory Policy North America organization in Bridgewater, NJ
- Eight months will be spent at FDA in the Division of Pediatrics and Maternal Health in the Office of New Drugs in Silver Spring, MD
For more information, please see this brochure.
Visit Rutgers Health Institute for Pharmaceutical Industry Fellowships for eligibility criteria and key application dates. Questions? Contact Carolyn.Seyss@pharmacy.rutgers.edu
For questions about FDA-Affiliated Pharmacy Fellowship Programs that cannot be addressed directly to a specific program above, contact FDA’s Division of Drug Information at pharmacy.fellowships@fda.hhs.gov.