Inspections Yield Valuable Results, Regardless of Classification
By: Michael C. Rogers, Associate Commissioner for Regulatory Affairs
Inspecting regulated manufacturers is a critical part of the FDA’s public health protection mission. Rigorous oversight of the industries we regulate is essential to ensuring the safety and efficacy of the broad range of FDA-regulated products that Americans rely on every day. This includes not only our nation’s food and prescription and over-the-counter drug supplies, but also medical devices, like pacemakers, cardiac stents, and joint implants, as well as vaccines, blood, plasma, tobacco, and more.
The FDA conducts inspections for many purposes: to verify firms’ compliance with applicable laws and regulations; monitor and respond to foodborne illness outbreaks; remove harmful (including fraudulent) products from the marketplace; and stop violative products, such as illicit drugs and contaminated foods, from entering our borders. Although inspections are a snapshot in time, they provide a comprehensive review of firm operations and can identify relevant issues that, if not corrected, could pose a risk to consumers and patients; therefore, inspection outcomes are extremely valuable to protect consumers and public health.
Four Types of Inspections: Surveillance, Follow-Up, For-cause, and Application-based
There are four basic types of inspections that our consumer safety officers (CSOs), commonly referred to as investigators, conduct to achieve our public health mission: surveillance, follow-up, for-cause, and application-based.
A surveillance inspection refers to our periodic oversight inspections to evaluate a firm’s current good manufacturing practices, which includes examining aspects of production like manufacturing controls, and processing and packing methods.
A follow-up inspection assesses corrections a firm has made in response to any previously discovered violations, or as a result of any samples our investigators collected that are found to be violative, or unsafe. If a follow-up inspection reveals the corrective actions have not been made or are ineffective, official agency enforcement actions (such as product seizures or injunctions) may also be taken at this time.
A for-cause inspection determines whether a firm's actions in response to specific issues, including consumer complaints, product recalls, or foodborne illness or other adverse event outbreaks involving its products are appropriate and adequate for protecting public health.
Finally, an application-based inspection determines regulatory compliance of firms prior to the approval of their applications for a new medical device, drug, or biologic (vaccines, blood, living tissues, and gene therapies, for example) and ensures the firm has an adequate facility and the appropriate safety, sterility, and other procedures in place to manufacture the product precisely as described in its application.
Understanding the Inspection Process
The FDA uses a risk-based approach to identify and prioritize foreign and domestic facilities for inspection. This allows the efficient and effective use of FDA resources to address issues that may affect public health. ORA’s highly trained investigators focus on identifying potential issues during an inspection that could cause harm or danger to consumers. Upon arriving at an FDA-regulated facility, the investigator presents their credentials and issues a “Notice of Inspection” (FDA Form 482), then initiates a thorough inspection of the firm’s operations, including evaluating manufacturing practices and safety procedures, and discussing those findings with firm employees, as well as reviewing and collecting records, and documenting any observations. At the inspection's conclusion, the CSO discusses observations and concerns with firm management and may issue a written list of observations. Observations are meant to reveal potential violations, but do not represent a final agency determination of violations at a facility. There is still more work to do.
The Significance of Establishment Inspection Reports
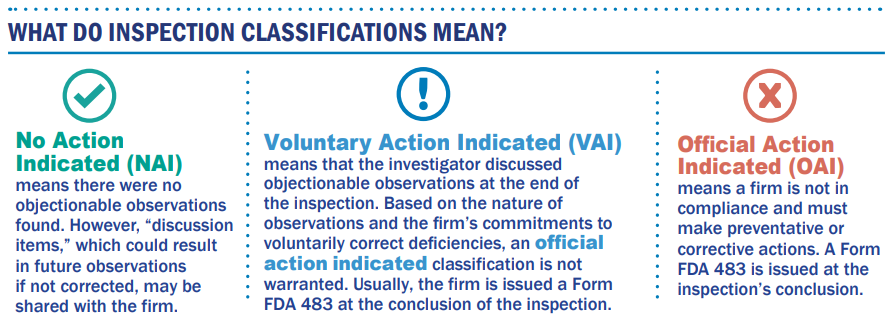
After the inspection is concluded, the CSO writes a detailed establishment inspection report, or EIR, that further explains any observations made during the inspection and provides documentation and evidence to support the observation. The agency evaluates the EIR and other submitted documentation to assign an inspection classification that reflects the firm’s compliance status at the time of the inspection. We then classify the inspection as one of the following:
The classification assignment process is a thoughtful and measured approach that ensures all factors are appropriately considered when making critical regulatory decisions. And it is absolutely vital that our decisions are accurate, fully informed, and science- and evidence-based. However, we still must maintain the ability to make decisions, even in the absence of information we would prefer to have. It is a delicate balancing act. As such, inspections serve as a critical snapshot in time, one of the many tools the agency uses to provide regulatory oversight over firms subject to FDA's requirements--including, significantly, when our CSOs find deficiencies and follow through with firms to ensure appropriate actions are taken to protect the public from harm. But the NAI, or No Action Indicated, finding is also revealing--both to the agency and to consumers. This classification conveys the important message that a firm was inspected by a world-class inspectorate and no significant objectionable conditions were observed. Firms in this category can be seen as “generally in compliance with FDA’s regulatory requirements at the time of the inspection.”
Inspection Benefits Firms
In addition, inspections, at a minimum, also serve as an educational tool for firms. Firm representatives can accompany CSOs during their inspection of a facility, while answering and asking questions, readily providing requested records, and making immediate voluntary corrective actions. Firms can also use the EIR to proactively address potential issues, correct deficiencies, and make procedural changes to strengthen their operations and build consumer trust. These actions are positive indications of a firm’s desire to provide products of utmost safety and quality to Americans.
The Value of Inspections
You may be asking yourself: Why are inspections that don’t identify violations still valuable?
Inspections are critical to the FDA’s oversight, even those in which no objectionable observations have been found. That’s because the FDA relies upon firms to ensure their products meet regulations and appreciates firms that achieve or exceed regulatory requirements. A firm’s ability to readily comply with laws and regulations signals to the agency that the firm prioritizes safety and takes public health seriously. Firms, who, during an inspection, can demonstrate good manufacturing practices, good employee hygiene, well-trained staff, quality controls, and show a willingness to proactively implement corrective or preventive actions to achieve or exceed standards –are emblematic of what the FDA wants to see in its quest to continue safeguarding Americans from potentially harmful, unsafe, and ineffective products.