Dermal Filler Do's and Don'ts for Wrinkles, Lips and More
Check out the FDA's tips to safely use dermal fillers and learn the difference between dermal fillers and injectable botulinum toxin products.
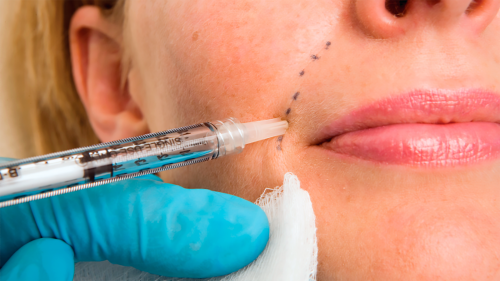
People are seeking treatments to smooth smile lines and crow’s feet and plump up their lips, cheeks, and hands.
Injecting dermal fillers into the face and hands can improve the appearance of facial lines and volume loss caused by age or certain medical conditions. In studies of dermal fillers approved by the U.S. Food and Drug Administration, people generally report they are satisfied with their treatment results.
However, dermal fillers are not for everyone. Dermal fillers may not be appropriate for people with certain conditions, such as bleeding disorders or some allergies. If your health care provider confirms that dermal fillers are an option for you, know that all medical products have benefits and risks. The FDA advises you work with a licensed health care provider who is experienced in injecting dermal fillers, knowledgeable about fillers, anatomy, managing complications, and most importantly, tells you about the risks and benefits before receiving treatment.
What are dermal fillers?
Dermal fillers are gel-like substances injected under the skin. Dermal fillers are meant to create a smoother or fuller appearance, or both.
The FDA regulates dermal fillers as medical devices. As reported in clinical trials, the effects of most FDA-approved dermal fillers are temporary because they are made from materials that the body eventually breaks down and absorbs. The injection procedure may have to be repeated to maintain the desired effect.
Types of dermal fillers
Temporary fillers include the following materials:
- Hyaluronic acid, a sugar that is naturally found in the body
- Calcium hydroxylapatite, a mineral and a major component of bone
- Poly-L-lactic acid (PLLA), a biodegradable, synthetic material
There’s only one FDA-approved dermal filler that is not absorbed by the body. It is made with polymethylmethacrylate (PMMA) beads suspended in a solution that contains bovine (cow) collagen. PMMA beads are tiny round, smooth, plastic beads.
FDA-approved uses of dermal fillers
Dermal fillers are approved for specific uses in people aged 22 and older. Those uses include:
- Correcting moderate-to-severe facial wrinkles and skin folds
- Increasing fullness of lips, cheeks, chin, under-eye hollows, jawline, and back of the hand
- Restoring facial fat loss in people with human immunodeficiency virus (HIV)
- Correcting acne scars on the cheek
FDA warnings about unapproved fillers
- The FDA has not approved injectable silicone or any injectable fillers for body contouring or enhancement. The FDA has warned against getting filler injected into the breasts, buttocks, or spaces between the muscles. Using injectable filler for large-scale body contouring or body enhancement can lead to serious injury, including long-term pain, infection, permanent scarring or disfigurement, and even death.
- The FDA has not approved needle-free devices for the injection of dermal fillers and warns against using them to inject hyaluronic acid or other lip and facial fillers. The injectors use high pressure and do not provide enough control over where filler will be placed. Serious injuries and in some cases, permanent harm to the skin, lips or eyes have occurred.
- The FDA also warns against buying or using lip or facial fillers that are sold directly to the public. They are not FDA approved and may be contaminated with chemicals and infectious organisms. The only FDA-approved dermal fillers are supplied by a prescription for injection by a licensed health care professional using a syringe with a needle or a cannula (a small flexible tubing with a blunt tip that is inserted under the skin).
Risks of FDA-approved fillers
As with any medical procedure, there are risks involved with the use of dermal fillers. Most side effects associated with dermal fillers, such as swelling and bruising occur shortly after injection and many resolve in a few days to weeks. In some cases, side effects may emerge weeks, months, or years later.
Common risks include:
- Bruising
- Redness
- Swelling
- Pain
- Tenderness
- Itching
- Rash
- Difficulty in performing activities (only observed when injected into the back of the hand)
Less common risks include:
- Inflammation such as swelling or redness may develop near the dermal filler injection site following viral or bacterial illnesses or infections, vaccinations, or dental procedures
- Raised bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgically removed
- Infection
- Open or draining wounds
- A sore at the injection site
- Allergic reaction
- Necrosis (tissue death)
People should be tested for allergies before receiving dermal fillers made with certain materials, especially materials derived from animals, such as collagen.
Unintended injection into blood vessels
The most serious risk associated with dermal fillers is accidental injection into a blood vessel. Filler that enters a blood vessel can cause skin necrosis (death of tissue), stroke, or blindness. While the chances of this happening are low, if it does happen, the resulting complications can be serious and may be permanent.
Removing Dermal Fillers
If you want to have fillers removed or reduced because of side effects, you may need additional procedures to reduce the filler or surgery to remove it. These procedures carry their own risks. Be aware that it may be difficult or impossible to remove some filler materials.
6 Tips for Consumers About Injectable Dermal Fillers
- Do work with a licensed health care provider who has experience in the fields of dermatology or plastic surgery and is trained to inject dermal fillers. The provider should use properly labeled, sealed vials or pre-filled syringes of FDA-approved filler.
- Do request and read the patient labeling information on FDA-approved injectable dermal fillers from your licensed health care provider.
- Do know the type of product to be injected and the possible risks. Know where each product you will be receiving is to be injected. Talk to your licensed health care provider if you have any questions.
- Do not buy dermal fillers that are sold directly to the public. They may be fake, contaminated, or not approved for use in the U.S. FDA-approved dermal fillers are indicated for prescription use only.
- Do not inject yourself with dermal fillers or with needle-free injection “pens.”
- Do not get any type of filler or liquid silicone injected for body contouring.
Dermal Fillers and Botulinum Toxin Products
The FDA also has approved botulinum toxin products such as Botox, Dysport, Xeomin and Jeuveau to treat facial wrinkles. These products are not dermal fillers. They are injectable drugs that work by keeping muscles from tightening, so the wrinkles don’t show as much. The safe use of dermal fillers in combination with Botox and other treatments has not been evaluated in clinical studies.
Although botulinum toxin products are derived from the same bacteria that cause botulism, the amounts used for cosmetic purposes are purified and many orders of magnitude smaller.
The FDA has approved these injectable drugs for the temporary improvement in the appearance of one, or perhaps several types of facial lines, including frown lines, forehead lines, and crow’s feet.
Side effects reported in clinical trials include facial weakness, eyelid drooping, and brow drooping. Other adverse events included localized pain, swelling, reddening, and bruising at the injection site. In rare cases, injections have resulted in double vision, dry eyes, or difficulty swallowing or breathing. The injection of botulinum toxin products for cosmetic purposes is not recommended for use while pregnant or lactating.
Additional Information
If you have experienced a problem with a dermal filler product or other product regulated by the FDA, you can voluntarily report it to MedWatch, the FDA safety information and adverse event reporting program.
