Drug Trial Snapshot: Ozempic
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the OZEMPIC Package Insert for complete information.
OZEMPIC (semaglutide)
(oh-ZEM-pick)
Novo Nordisk Inc.
Approval date: December 5, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OZEMPIC is a drug that improves blood sugar control in adults with type 2 diabetes mellitus (DM) when used in addition to diet and exercise.
How is this drug used?
OZEMPIC is available as a liquid that comes in a prefilled pen. It is injected once weekly under the skin (subcutaneously) of the abdomen, thigh or upper arm. OZEMPIC may be used alone or in combination with other FDA-approved diabetes medications such as metformin, sulfonylureas, thiazolidinedione and insulin. In patients also using insulin injections, OZEMPIC and insulin should be injected separately and not mixed.
What are the benefits of this drug?
In patients with type 2 diabetes, treatment with OZEMPIC can lower HbA1c (hemoglobin A1c), which is a measure of blood sugar control.
What are the benefits of this drug (results of trials used to assess efficacy)?
The FDA considered the results of five randomized, multinational clinical trials to evaluate the efficacy of OZEMPIC in the treatment of adult patients with type 2 diabetes mellitus. In these trials, OZEMPIC was used as monotherapy, or in combination with oral antidiabetic medications with or without basal insulin, and compared to placebo, sitagliptin, exenatide extended release and insulin glargine. The primary efficacy endpoint in each trial was change in HbA1c from baseline. The results of each trial are presented in the tables below.
Table 3: Results at Week 30 in a Trial of OZEMPIC as Monotherapy in Adult Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Diet and Exercise in the Intent-to-Treat (ITT) Population – SUSTAIN 1 Trial
| Placebo | OZEMPIC 0.5 mg | OZEMPIC 1 mg | |
|---|---|---|---|
| ITT Population (N)a | 129 | 128 | 130 |
| HbA1c (%) | |||
| Baseline (mean) | 8.0 | 8.1 | 8.1 |
| Change at Week 30b | -0.1 | -1.4 | -1.6 |
| Difference from placebo (95% CI)b/th> | -1.2 (-1.5, - 0.9)c | -1.4 (-1.7, -1.1)c | |
| Patients (%) achieving HbA1c <> | 28 | 73 | 70 |
| FPG (mg/dL) | |||
| Baseline (mean) | 174 | 174 | 179 |
| Change at Week 30b | -15 | -41 | -44 |
aITT population included all randomized and exposed patients. At week 30, the primary efficacy endpoint HbA1C was missing for 10%, 7% and 7% of patients and during the trial, rescue medication was initiated by 20%, 5 and 4% of patients randomized to placebo, OZEMPIC 0.5 mg and OZEMPIC 1 mg. Missing data were imputed using multiple imputation based on retrieved dropouts.
bIntent to treat analysis using ANCOVA adjusted for baseline value and country
cp<0.0001 (2-sided)="" for="" superiority,="" adjusted="" for=""
FDA prescribing Information
Table 4: Results at Week 56 in a Trial of OZEMPIC Compared to Sitagliptin in Patients with Type 2 Diabetes Mellitus in Combination with Metformin and/or Thiazolidinediones – SUSTAIN 2 Trial
| OZEMPIC 0.5 mg | OZEMPIC 1 mg | Sitagliptin | |
|---|---|---|---|
| ITT Population (N)a | 409 | 409 | 407 |
| HbA1c (%) | |||
| Baseline (mean) | 8.0 | 8.0 | 8.2 |
| Change at week 56b | -1.3 | -1.5 | -0.7 |
| Difference from sitagliptin (95% CI)b | -0.6 (-0.7, -0.4)c | -0.8 (-0.9, -0.6)c | |
| Patients achieving HbA1c <> | 66 | 73 | 40 |
| FPG (mg/dL) | |||
| Baseline (mean) | 168 | 167 | 173 |
| Change at week 56b | -35 | -43 | -23 |
aITT population included all randomized and exposed patients. At week 56, the primary efficacy endpoint HbA1C was missing for 7%, 5% and 6% of patients and during the trial, rescue medication was initiated by 5%, 2% and 19% of patients randomized to OZEMPIC 0.5 mg, OZEMPIC 1 mg and sitagliptin respectively. Missing data were imputed using multiple imputation based on retrieved dropouts.
bIntent to treat analysis using ANCOVA adjusted for baseline value and country
cp<0.0001 (2-sided)="" for="" superiority,="" adjusted="" for=""
FDA prescribing Information
Table 5: Results at Week 56 in a Trial of OZEMPIC Compared to Exenatide 2 mg once-weekly in Adult Patients with Type 2 Diabetes Mellitus In Combination with Metformin or Metformin with Sulfonylurea – SUSTAIN 3 Trial
| OZEMPIC 1 mg | Exenatide ER 2 mg | |
|---|---|---|
| ITT Population (N)a | 404 | 405 |
| HbA1c (%) | ||
| Baseline (mean) | 8.4 | 8.3 |
| Change at week 56b | -1.4 | -0.9 |
| Difference from exenatide (95% CI)b | -0.5 (-0.7, -0.3)c | |
| Patients achieving HbA1c <> | 62 | 40 |
| FPG (mg/dL) | ||
| Baseline (mean) | 191 | 188 |
| Change at week 56b | -44 | -34 |
aITT population included all randomized and exposed patients. At week 56, the primary efficacy endpoint HbA1C was missing for 9% and 11% of patients and during the trial, rescue medication was initiated by 5% and 10% of patients randomized to OZEMPIC 1 mg and exenatide ER respectively. Missing data were imputed using multiple imputation based on retrieved dropouts.
bIntent to treat analysis using ANCOVA adjusted for baseline value and country
cp<0.0001 (2-sided)="" for="" superiority,="" adjusted="" for=""
FDA prescribing Information
Table 6: Results at Week 30 in a Trial of OZEMPIC Compared to Insulin Glargine in Adult Patients with Type 2 Diabetes Mellitus In Combination with Metformin or Metformin with Sulfonylurea – SUSTAIN 4 Trial
| OZEMPIC0.5 mg | OZEMPIC1 mg | Insulin Glargine | |
|---|---|---|---|
| ITT Population (N)a | 362 | 360 | 360 |
| HbA1c (%) | |||
| Baseline (mean) | 8.1 | 8.2 | 8.1 |
| Change at week 30b | -1.2 | -1.5 | -0.9 |
| Difference from insulin glargine (95% CI)b | -0.3 (-0.5, -0.1)c | -0.6 (-0.8, -0.4)c | |
| Patients achieving HbA1c <> | 57 | 66 | 40 |
| FPG (mg/dL) | |||
| Baseline (mean) | 172 | 179 | 174 |
| Change at week 30b | -35 | -46 | -37 |
aITT population included all randomized and exposed patients. At week 30, the primary efficacy endpoint HbA1c was missing for 8%, 6% and 6% of patients and during the trial, rescue medication was initiated by 4%, 3% and 1% of patients randomized to OZEMPIC 0.5 mg, OZEMPIC 1 mg and insulin glargine respectively. Missing data were imputed using multiple imputation based on retrieved dropouts.
bIntent to treat analysis using ANCOVA adjusted for baseline value, country and stratification factors
cp<0.0001 (2-sided)="" for="" superiority,="" adjusted="" for=""
FDA prescribing Information
Table 7: Results at Week 30 in a Trial of OZEMPIC in Adult Patients with Type 2 Diabetes Mellitus In Combination with Basal Insulin With or Without Metformin – SUSTAIN 5
| Placebo | OZEMPIC 0.5 mg | OZEMPIC 1 mg | |
|---|---|---|---|
| ITT Populationa | 133 | 132 | 131 |
| HbA1c (%) | |||
| Baseline (mean) | 8.4 | 8.4 | 8.3 |
| Change at week 30b | -0.2 | -1.3 | -1.7 |
| Difference from placebo (95% CI)b | -1.1 (-1.4, -0.8)c | -1.6 (-1.8, -1.3)c | |
| Patients achieving HbA1c <> | 13 | 56 | 73 |
| FPG (mg/dL) | |||
| Baseline (mean) | 154 | 161 | 153 |
| Change at week 30b | -8 | -28 | -39 |
aITT population included all randomized and exposed patients. At week 30, the primary efficacy endpoint HbA1c was missing for 7%, 5% and 5% of patients and during the trial, rescue medication was initiated by 14%, 2% and 1% of patients randomized to placebo, OZEMPIC 0.5 mg and OZEMPIC 1 mg respectively. Missing data were imputed using multiple imputation based on retrieved dropouts.
bIntent to treat analysis using ANCOVA adjusted for baseline value, country and stratification factors
cp<0.0001 (2-sided)="" for="" superiority,="" adjusted="" for=""
FDA prescribing Information
A separate randomized, double-blind, placebo-controlled, non-inferiority trial evaluated cardiovascular (CV) outcomes during treatment with OZEMPIC in patients with type 2 diabetes mellitus at high risk for cardiovascular events. The primary composite cardiovascular endpoint was the time to first occurrence of a major adverse cardiovascular event (MACE), which included cardiovascular death, myocardial infarction, or stroke. No increased risk for MACE was observed with OZEMPIC.
Table 8: Cardiovascular Outcomes – SUSTAIN 6 Trial
| PlaceboN = 1649 | OZEMPICN = 1648 | |
|---|---|---|
| Primary composite CV Event | ||
| N of patients with CV event (%) | 146 (8.9) | 108 (6.6) |
| Total person year | 3401.1 | 3408.2 |
| Incidence rate per 100 PY | 4.3 | 3.2 |
FDA Statistical Review
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Overall, OZEMPIC worked similarly in men and women
- Race: OZEMPIC worked similarly in White, Black/African American and Asian races. The number of patients of other races was small; therefore, differences in response could not be determined.
- Age: OZEMPIC worked similarly in patients younger or older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Tables 9, 10, 11, and 12 summarize the effect of OZEMPIC on HbA1c by subgroup in the clinical trials.
Table 9. Effects of OZEMPIC on HbA1c by subgroups (Placebo-Controlled Trials – SUSTAIN 1 and 5)
| Demographic Parameters | OZEMPIC 0.5 mg | OZEMPIC 1 mg | Placebo | Treatment Difference (OZEMPIC 0.5 mg - Placebo) [95% CI] | Treatment Difference (OZEMPIC 1.0 mg - Placebo) [95% CI] | |||
|---|---|---|---|---|---|---|---|---|
| N | LS Mean Change from Baseline to Week 30 | N | LS Mean Change from Baseline to Week 30 | N | LS Mean Change from Baseline to Week 30 | |||
| Sex | ||||||||
| Male | 134 | -1.38 | 157 | -1.72 | 141 | -0.16 | -1.21 [-1.515, -0.912] | -1.56 [-1.843, -1.287] |
| Female | 126 | -1.29 | 104 | -1.58 | 121 | -0.17 | -1.11 [-1.42, -0.791] | -1.41 [-1.728, -1.084] |
| Age Group | ||||||||
| below 65 years | 195 | -1.36 | 212 | -1.7 | 191 | -0.11 | -1.25 [-1.501, -0.997] | -1.58 [-1.825, -1.345] |
| 65 years and above | 65 | -1.27 | 49 | -1.5 | 71 | -0.29 | -0.98 [-1.419, -0.546] | -1.22 [-1.67, -0.777] |
| Race | ||||||||
| White | 191 | -1.27 | 186 | -1.53 | 179 | -0.21 | -1.07 [-1.326, -0.806] | -1.33 [-1.574, -1.076] |
| Black or African American | 15 | -1.72 | 20 | -1.74 | 17 | 0.48 | -2.23 [-3.258, -1.211] | -2.23 [-3.139, -1.315] |
| Asian | 45 | -1.58 | 48 | -2.09 | 56 | -0.16 | -1.42 [-1.873, -0.975] | -1.92 [-2.369, -1.47] |
| Ethnicity | ||||||||
| Hispanic or Latino | 49 | -0.98 | 57 | -1.25 | 55 | -0.24 | -0.75 [-1.25, -0.246] | -1.03 [-1.509, -0.546] |
| Not Hispanic or Latino | 211 | -1.43 | 204 | -1.77 | 207 | -0.15 | -1.28 [-1.519, -1.044] | -1.62 [-1.852, -1.391] |
FDA Statistics Review
Table 10. Effects of OZEMPIC on HbA1c Compared to Sitagliptin by Subgroup - SUSTAIN 2 Trial
| Demographic parameter | OZEMPIC 0.5 mg | OZEMPIC 1 mg | Sitagliptin | Treatment Difference (OZEMPIC 0.5 mg - Sitagliptin) [95% CI] | Treatment Difference (OZEMPIC 1 mg - Sitagliptin) [95% CI] | |||
|---|---|---|---|---|---|---|---|---|
| N | LS Mean Change from Baseline to Week 56 | N | LS Mean Change from Baseline to Week 56 | N | LS Mean Change from Baseline to End of Study | |||
| Sex | ||||||||
| Male | 207 | -1.31 | 205 | -1.47 | 208 | -0.76 | -0.55 [-0.761, -0.337] | -.071 [-0.914, -0.503] |
| Female | 202 | -1.27 | 204 | -1.49 | 199 | -0.67 | -0.59 [-0.812, -0.373] | -0.82 [-1.037, -0.601] |
| Age Category | ||||||||
| Below 65 years | 333 | -1.3 | 332 | -1.49 | 328 | -0.69 | -0.61 [-0.786, -0.444] | -0.8 [-0.972, -0.636] |
| 65 years and older | 76 | -1.23 | 77 | -1.44 | 79 | -0.85 | -0.39 [-0.736, -0.035] | -0.59 [-0.934, -0.253] |
| Race | ||||||||
| White | 279 | -1.27 | 279 | -1.47 | 281 | -0.72 | -0.55 [-0.735, -0.368] | -0.75 [-0.933, -0.57] |
| Black or African American | 18 | -1.41 | 24 | -1.56 | 17 | -0.86 | -0.56 [-1.39, 0.279] | -0.7 [-1.429, 0.028] |
| Asian | 106 | -1.31 | 99 | -1.44 | 102 | -0.7 | -0.61 [-0.907, -0.315] | -0.74 [-1.038, -0.45] |
| Ethnicity | ||||||||
| Hispanic or Latino | 69 | -1.47 | 67 | -1.57 | 73 | -0.74 | -0.72 [-1.074, -0.37] | -0.83 [-1.177, -0.48] |
| Not Hispanic or Latino | 340 | -1.25 | 342 | -1.46 | 334 | -0.71 | -0.54 [-0.709, -0.367] | -0.75 [-0.916, -0.58] |
FDA Statistical Review
Table 11. Effects of OZEMPIC on HbA1c Compared to Exenatide by Subgroup– SUSTAIN 3 Trial
| Demographic parameter | OZEMPIC 1 mg | Exenatide | Treatment Difference (OZEMPIC 1 mg - Exenatide) [95% CI] | ||
|---|---|---|---|---|---|
| N | LS Mean Change from Baseline to Week 56 | N | LS Mean Change from Baseline to Week 56 | ||
| Sex | |||||
| Male | 219 | -1.28 | 228 | -0.89 | -0.38 [-0.638, 0.131] |
| Female | 185 | -1.53 | 177 | -0.92 | -0.6 [0.873, 0.333] |
| Age Group | |||||
| Below 65 years | 316 | -1.4 | 298 | -0.9 | -0.49 [-0.708, -0.275] |
| 65 years and older | 88 | -1.38 | 107 | -0.9 | -0.48 [0.844, 0.113] |
| Race | |||||
| White | 341 | -1.44 | 338 | -0.95 | -0.49 [-0.696, -0.292] |
| Black or African American | 28 | -0.86 | 30 | -0.4 | -0.45 [-1.145, 0.236] |
| Asian | 8 | -1.26 | 6 | -0.89 | -0.36 [-1.169, 0.209] |
| Ethnicity | |||||
| Hispanic or Latino | 91 | -1.14 | 106 | -0.57 | -0.58[-0.943, -0.213] |
| Not Hispanic or Latino | 313 | -1.48 | 299 | -1.01 | -0.46 [-0.675, -0.247] |
FDA Statistical Review
Table 12. Effects of OZEMPIC on HbA1c Compared to Insulin Glargine by Subgroup– SUSTAIN 4 Trial
| Demographic parameter | OZEMPIC 0.5 | OZEMPIC 1 mg | Insulin Glargine | Treatment Difference (OZEMPIC 0.5 mg – Insulin Glargine) [95% CI] | Treatment Difference (OZEMPIC 1 mg –Insulin Glargine) [95% CI] | |||
|---|---|---|---|---|---|---|---|---|
| N | LS Mean Change from Baseline to Week 30 | N | LS Mean Change from Baseline to Week 30 | N | LS Mean Change from Baseline to Week 30 | |||
| Sex | ||||||||
| Male | 197 | -1.13 | 182 | -1.55 | 195 | -1.02 | -0.11 [-0.362, 0.14] | -0.53 [-0.788, -0.275] |
| Female | 165 | -1.19 | 178 | -1.41 | 165 | -0.73 | -0.45 [-0.687, -0.221] | -0.67 [-0.91, -0.44] |
| Age Category | ||||||||
| Below 65 years | 278 | -1.16 | 281 | -1.59 | 281 | -0.89 | -0.27 [-0.474, -0.065] | -0.7 [-0.906, -0.495] |
| 65 years and older | 84 | -1.15 | 79 | -1.11 | 79 | -0.9 | -.25 [-0.592, 0.082] | -0.21 [-0.56, 0.132] |
| Race | ||||||||
| White | 279 | -1.18 | 279 | -1.51 | 276 | -0.89 | -0.3 [-0.49, -0.102] | -0.62 [-0.815, -0.425] |
| Black or African American | 32 | -1.17 | 34 | -1.65 | 33 | -1.09 | -0.08 [-0.614, 0.451] | -0.56 [-1.105, -0.014] |
| Asian | 42 | -0.88 | 39 | -1.23 | 38 | -0.71 | -0.16 [-0.687, 0.361] | -0.51 [-1.053, 0.023] |
| Ethnicity | ||||||||
| Hispanic or Latino | 61 | -1.16 | 74 | -1.44 | 78 | -0.73 | -0.43 [-0.801, -0.054] | -0.71 [-1.079, -0.342] |
| Not Hispanic or Latino | 301 | -1.15 | 286 | -1.49 | 281 | -0.94 | -0.21 [-0.404, -0.008] | -0.55 [-0.751, -0.35] |
FDA Statistical Review
What are the possible side effects?
OZEMPIC may cause serious side effects including low blood sugar, inflammation of the pancreas, complications of diabetes-related retina disease (diabetic retinopathy) and allergic reactions. In animal studies, mice and rats that received OZEMPIC were more likely to develop a certain kind of thyroid cancer. It is not known whether this may occur in humans.
The most common side effects in clinical trials included nausea, vomiting, diarrhea, abdominal pain and constipation.
What are the possible side effects (results of trials used to assess safety)?
Table 13 summarizes common adverse reactions (excluding hypoglycemia) from two placebo-controlled trials. OZEMPIC was used alone in one trial and in combination with basal insulin in the other trial.
Table 13: Adverse Reactions Reported in ≥5% of OZEMPIC-Treated Patients with Type 2 Diabetes Mellitus in Two Placebo-Controlled Trials
| Adverse Reaction | Placebo (N = 262) % |
OZEMPIC 0.5 mg (N = 260) % |
OZEMPIC 1 mg (N = 261) % |
|---|---|---|---|
| Nausea | 6.1 | 15.8 | 20.3 |
| Vomiting | 2.3 | 5.0 | 9.2 |
| Diarrhea | 1.9 | 8.5 | 8.8 |
| Abdominal pain | 4.6 | 7.3 | 5.7 |
| Constipation | 1.5 | 5.0 | 3.1 |
FDA Prescribing Information
The types and frequency of common adverse reactions, excluding hypoglycemia, in other trials were similar to those listed in Table 13.
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar between men and women.
- Race: The risk of side effects was similar between White, Black/African American and Asian races. The number of patients of other races was small; therefore, differences could not be determined.
- Age: The risk of side effects was similar between patients younger or older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The frequency of gastrointestinal adverse reactions in the two placebo-controlled trials by sex, age, and race are shown in Table 14.
Table 14: Frequency of Gastrointestinal Adverse Reactions in Placebo-Controlled Trials By Sex, Race, and Age
| Demographic Parameter | Placebo N = 262 | OZEMPIC 0.5 and 1 mg N = 521 |
|---|---|---|
| Sex | ||
| Men | 17/141 (12.1%) | 91/291 (31.3%) |
| Women | 17/121 (14.0%) | 76/230 (33.0%) |
| Race | ||
| White | 23/179 (12.8%) | 129/377 (34.2%) |
| Black or African American | 4/17 (23.5%) | 7/35 (20.0%) |
| Asian | 6/56 (10.7%) | 3/93 (3.0%) |
| Age Category | ||
| 18-64 years | 25/191 (13.1%) | 123/407 (30.2%) |
| ≥65 years | 9/71 (12.6%) | 44/114 (38.6%) |
Clinical Trial Data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved OZEMPIC based on evidence from seven clinical trials of 4087 patients with type 2 DM. The trials were conducted at 536 sites in 33 countries, including Canada, Mexico, Russian Federation, Ukraine, Turkey, India, South Africa, Japan, Hong Kong, multiple European countries, Argentina, and the United States.
The FDA also considered data from one separate trial of 3297 patients with type 2 DM who were at high risk for cardiovascular events. This trial was conducted in 20 countries in Europe, Russian Federation, Turkey, Brazil, Israel, Malaysia, Brazil, Mexico, Thailand, Taiwan, Canada, and the United States.
These two populations will be presented separately.
Figures 1, 2 and 3 summarize the number patients in the 7 clinical trials by sex, race, and age.
Figure 1: Baseline Demography by Sex – Safety Population
Clinical Trial Data
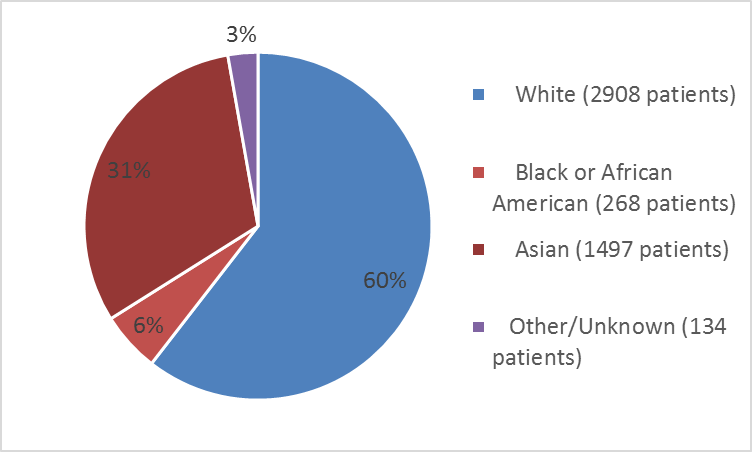
Figure 2: Baseline Demography by Race – Safety Population
Clinical Trial Data
Table 1: Baseline Demography by Race – Safety Population
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2908 | 60 |
| Black or African American | 268 | 6 |
| Asian | 1497 | 31 |
| American Indian or Native Alaskan | 6 | less than 1 |
| Hawaiian or Other Pacific Islander | 3 | less than 1 |
| Other | 63 | 1 |
| Not reported | 62 | 1 |
Clinical Trial Data
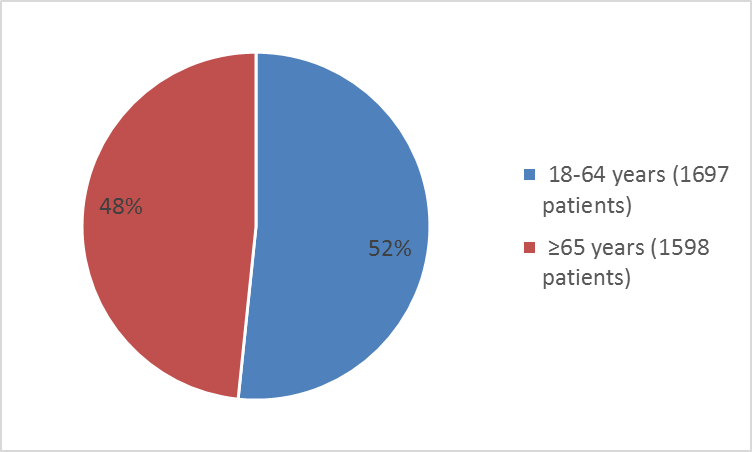
Figure 3: Baseline Demography by Age – Safety Population
Clinical Trial Data
Figures 4, 5, and 6 summarize the patients at high risk for cardiovascular events by sex, race, and age.
Figure 4: Baseline Demography by Sex – Patients at High Risk for CV Event – Safety Population
Clinical Trial Data
Figure 5: Baseline Demography by Race – Patients at High Risk for CV Event – Safety Population
Clinical Trial Data
Table 2: Baseline Demography by Race – Patients at High Risk for CV Events – Safety Population
|
Race |
Number of Patients | Percentage |
|---|---|---|
|
White |
2725 | 83 |
|
Black or African American |
221 | 7 |
|
Asian |
273 | 8 |
|
American Indian or Native Alaskan |
10 | less than 1 |
|
Hawaiian or Other Pacific Islander |
3 | less than 1 |
|
Other |
54 | 2 |
Clinical Trial Data
Figure 6: Baseline Demography by Age Category – Patients at High Risk for CV Events – Safety Population
Clinical Trial Data
Who Participated in the Trials?
Five clinical trials were included in the efficacy evaluation. These five trials included 3899 patients. Two additional trials in 908 patients were conducted in Japan and were included in the safety evalution, for a total of 4087 patients.
An additional trial in 3286 patients was conducted in patients with type 2 diabetes who were at high risk of cardiovascular events.
Table 15: Demographics of Safety Trials – Safety Population
| Demographic Parameter | Number of Patients N = 4807 | Percentage |
|---|---|---|
| Sex | ||
| Men | 2737 | 56.9 |
| Women | 2070 | 43.1 |
| Race | ||
| White | 2908 | 60.5 |
| Black or African American | 268 | 5.6 |
| Asian | 1497 | 31.2 |
| American Indian or Native Alaskan | 6 | <> |
| Hawaiian or Other Pacific Islander | 3 | <> |
| Other | 63 | 1.3 |
| Not reported | 62 | 1.3 |
| Ethnicity | ||
| Hispanic or Latino | 780 | 16.2 |
| Not Hispanic or Latino | 4027 | 83.8 |
| Age Category | ||
| 18-64 years | 3655 | 76.0 |
| ≥65 years | 1152 | 24.0 |
Clinical Trial Data
Table 16: Baseline Demographics of Trial in Patients at High Risk for Cardiovascular Events
| Demographic Parameter | Number of Patients N = 3286 | Percentage |
|---|---|---|
| Sex | ||
| Men | 1994 | 60.7 |
| Women | 1292 | 39.3 |
| Race | ||
| White | 2725 | 82.9 |
| Black or African American | 221 | 6.7 |
| Asian | 273 | 8.3 |
| American Indian or Native Alaskan | 10 | <> |
| Hawaiian or Other Pacific Islander | 3 | <> |
| Other | 54 | 1.6 |
| Ethnicity | ||
| Hispanic or Latino | 506 | 15.4 |
| Not Hispanic or Latino | 2780 | 84.6 |
| Age Category | ||
| 18-64 years | 1697 | 51.6 |
| ≥65 years | 1589 | 49.4 |
Clinical Trial Data
Table 17: Demographics of Efficacy Trials – Full Analysis Population
| Demographic Parameter | Number of Patients N = 3899 | Percentage |
|---|---|---|
| Sex | ||
| Men | 2073 | 53.2 |
| Women | 1826 | 46.8 |
| Race | ||
| White | 2908 | 74.6 |
| Black or African American | 268 | 6.9 |
| Asian | 589 | 15.1 |
| American Indian or Native Alaskan | 6 | <> |
| Hawaiian or Other Pacific Islander | 3 | <> |
| Other | 63 | 1.6 |
| Not Reported | 62 | 1.6 |
| Ethnicity | ||
| Hispanic or Latino | 780 | 20.0 |
| Not Hispanic or Latino | 3119 | 80.0 |
| Age Category | ||
| 18-64 years | 3045 | 78.1 |
| ≥ 65 years | 854 | 21.9 |
Clinical Trial Data
How were the trials designed?
The benefits and side effects of OZEMPIC for the treatment of adult patients with type 2 DM were evaluated in seven clinical trials. In two of these trials (NCT #02054897 and NCT#02305381), patients were randomly assigned to receive either OZEMPIC or placebo injection weekly. Neither the patient nor the health care provider knew which treatment was being given until after the trials were completed. Treatment was given for 30 weeks.
In the other five trials (NCT #01930188, 01885208, 02128932, 02207374, 02254291), patients were randomly assigned to receive either OZEMPIC or another antidiabetic medication, and the patient and provider knew which medication was being given in four trials. Treatment was given for 30 weeks or 56 weeks.
In each trial, HbA1c was measured from the start of the trial to the end of the trial and compared between the OZEMPIC group and the other groups.
There was also an additional trial where patients with type 2 diabetes who were at high risk for cardiovascular events (NCT #01720446) were randomly assigned to receive OZEMPIC or placebo. Neither the patient nor the health care provider knew which treatment was being given. Treatment was given for 104 weeks (2 years), and the occurrence of cardiovascular events, including heart attacks, strokes and hospitalization due to unstable angina (near heart attack) were recorded and compared in the two groups of patients.
How were the trials designed?
The efficacy of OZEMPIC was evaluated in 5 trials.
Two randomized, double-blind, placebo-controlled, multinational clinical trials evaluated the efficacy of two doses of OZEMPIC as monotherapy or in combination with basal insulin. The primary endpoint was change in HbA1C from baseline to week 30.
Three additional randomized, active-controlled, multinational, non-inferiority design clinical trials evaluated the efficacy of two doses of OZEMPIC added to other antidiabetic medications and compared to either sitagliptin, exenatide ER, or insulin glargine. The trial that compared OZEMPIC to sitagliptin was double-blind, whereas the other two active-controlled trials were open-label.
Safety of OZEMPIC was evaluated in the above five trials and two additional trials conducted in Japan. Both Japanese trials were randomized, open-label, active-controlled trials in which OZEMPIC was added to other antidiabetic medications.
An additional randomized, double-blind, placebo-controlled, multinational trial was conducted in patients at high risk of cardiovascular events. The primary endpoint was time to first occurrence of a major atherosclerotic cardiovascular event (MACE), defined as any of the following events: cardiovascular death, myocardial infarction, or stroke.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.