Drug Trials Snapshot: JEMPERLI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the JEMPERLI Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
JEMPERLI (dostarlimab-glxy)
(Jem-PER-lee)
Glaxosmithkline, LLC
Approval date: 04/22/2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
JEMPERLI is a prescription medicine used to treat adult females with uterine cancer (endometrial cancer). JEMPERLI may be used when:
- the tumor has been shown by a laboratory test to be mismatch repair deficient (dMMR), and
- the tumor has returned, or it has spread (advanced cancer), and
- you have received certain types of chemotherapy that did not work or are no longer working
How is this drug used?
JEMPERLI is given into the vein through an intravenous (IV) line. JEMPERLI is usually given every 3 weeks for the first 4 doses, and then beginning 3 weeks later, it is usually given every 6 weeks. The healthcare provider will decide how many treatments are needed.
What are the benefits of this drug?
In the trial, JEMPERLI 42.3% of patients had a decrease in the size of their tumors (29.6%), or their tumors could no longer be detected (12.7%). For most patients (93.3%), the response lasted more than 6 months.
JEMPERLI was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy results based on confirmed objective response rate (ORR), as assessed by independent central review (ICR), and ICR-assessed duration of response are presented below
Table 1. Efficacy Results in GARNET dMMR Endometrial Cancer Population
|
Endpoint |
JEMPERLI (N = 71) |
|---|---|
|
Confirmed Overall Response Rate |
|
|
ORR |
42.3% |
|
(95% CI) |
(30.6, 54.6) |
|
Complete response rate |
12.7% |
|
Partial response rate |
29.6% |
|
Duration of Response |
|
|
Median in months |
Not reached |
|
(range)a |
(2.6, 22.4+) |
|
Patients with duration ≥6 months |
93.3% |
a Median follow-up for duration of response was 14.1 months, measured from time of first response.
+ Ongoing at last assessment
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All patients were female since JEMPERLI is for the treatment of endometrial cancer.
- Race: The number of patients of races other than White was small; therefore, differences in how well JEMPERLI works among races could not be determined.
- Age: JEMPERLI worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table below summarizes efficacy results by subgroup. These exploratory analyses should be interpreted with caution.
Table 2: Subgroup Analysis of Objective Response Rate
|
Demographic Subgroup |
Objective Response Rate |
|---|---|
|
Race |
|
|
White |
41.4 (28.6, 55.1) |
|
Black |
100 (2.5, 100) |
|
Asian |
50.0 (1.3, 98.7) |
|
American Indian or Alaska native |
66.7 (9.4, 99.2) |
|
Not reported |
28.6 (3.7, 71.0) |
|
Age |
|
|
< 65 |
38.9 (23.1, 56.5) |
|
≥65 |
45.7 (28.8, 63.4) |
Adapted from FDA review.
What are the possible side effects?
JEMPERLI can cause serious and potentially deadly immune reactions including inflammation of the lungs, gut, liver, kidneys, hormonal glands and skin as well as infusion related reactions, can cause fetal harm, and should not be taken if you’re breastfeeding.
The most common adverse reactions are fatigue, nausea, diarrhea, anemia, and constipation.
What are the possible side effects (results of trials used to assess safety)?
The Table below summarizes side effects that occurred in patients with dMMR endometrial cancer in GARNET Trial. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
Table 3. Adverse Drug Reactions in ≥10% in Patients with dMMR Endometrial Cancer who Received JEMPERLI in GARNET Trial
|
Adverse Reaction |
JEMPERLI (N = 104) |
|
|---|---|---|
|
All Grades |
Grade 3 or 4 |
|
|
Blood and Lymphatic System |
|
|
|
Anemia |
24 |
13 |
|
Gastrointestinal |
|
|
|
Nausea |
30 |
0 |
|
Diarrhea |
26 |
1.9 |
|
Constipation |
20 |
0.9 |
|
Vomiting |
18 |
0 |
|
General and Administration Site |
|
|
|
Fatigue |
48 |
1 |
|
Infections |
|
|
|
Urinary tract infection |
13 |
1.9 |
|
Metabolism and Nutrition |
|
|
|
Decreased appetite |
14 |
0 |
|
Musculoskeletal and Connective Tissue |
|
|
|
Myalgia |
12 |
0 |
|
Respiratory, Thoracic, and Mediastinal |
|
|
|
Cough |
14 |
0 |
|
Skin and Subcutaneous Tissue |
|
|
|
Pruritus |
14 |
1 |
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: All patients were female since JEMPERLI is for the treatment of endometrial cancer.
- Race: The number of patients of races other than White was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved JEMPERLI based on evidence from the GARNET trial (NCT02715284) of 71 patients with advanced or recurrent endometrial cancer that was shown to be mismatch repair deficient (dMMR), and for which certain types of chemotherapy did not work or was no longer working. The cohort used for the approved indication was conducted at 40 sites in 7 countries in North America and Europe.
Figures below summarize how many patients were enrolled in the clinical trial used to evaluate the efficacy of JEMPERLI by sex, race and age. There was no difference in patient demographics between the efficacy and safety populations.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
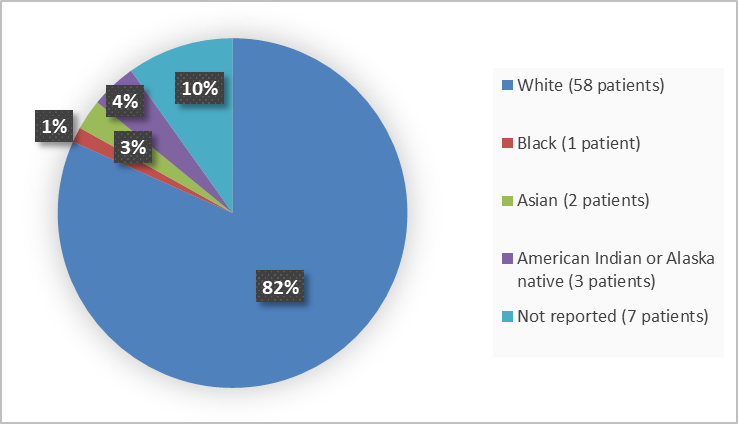
Figure 2. Baseline Demographics by Race (Efficacy Population)
Figure 3. Baseline Demographics by Age (Efficacy Population)
Who participated in the trials?
Table 4. Baseline Demographics by Age and Race (efficacy population)
|
Demographic |
dMMR EC n=71 (%) |
|---|---|
|
Age (years) |
|
|
Median, y |
64.0 |
|
Range, y |
39, 80 |
|
<65 yrs, n |
36 (51.0) |
|
≥65 yrs - <75 yrs |
28 (49.0) |
|
≥75 yrs |
7 (10.0) |
|
Race n(%) |
|
|
White |
58 (81.7) |
|
Black |
1 ( 1.4) |
|
Asian |
2 ( 2.8) |
|
American Indian or Alaska Native |
3 ( 4.2) |
|
Not reported |
7 (9.9) |
How were the trials designed?
The FDA approved JEMPERLI based on evidence from one clinical trial of 71 patients with advanced or recurrent endometrial cancer that was shown to be mismatch repair deficient (dMMR), and for which certain types of chemotherapy did not work or was no longer working.
The trial measured the proportion of patients who experienced partial shrinkage or complete disappearance of their tumors while receiving JEMPERLI.
How were the trials designed?
The efficacy and safety of JEMPERLI in patients with advanced or recurrent endometrial cancer that are mismatch repair deficient (dMMR) and that has progressed on or following prior treatment with a platinum-containing regimen were evaluated in one open-label, single-arm, non-randomized, multicohort trial.
The primary efficacy outcome measure of the trial was objective response rate (ORR) and duration of response (DoR). ORR was determined by blinded central review according to Response Evaluation Criteria in Solid Tumors (RECIST).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.