Drug Trial Snapshots: CYTALUX
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the CYTALUX Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
CYTALUX (pafolacianine)
(sahy-tuh-luhks)
On Target Laboratories, Inc.
Approval date: November 19, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CYTALUX is an optical imaging agent that is used as an adjunct in patients with ovarian cancer undergoing debulking surgery.
How is this drug used?
CYTALUX is injected intravenously as a single dose prior to surgery for use with near infrared imaging devices.
Who participated in the clinical trials?
The FDA approved CYTALUX based on evidence from 1 clinical trial of 150 patients with ovarian cancer. The trials were conducted at 10 sites in the United States and 1 site in the Netherlands.
What are the benefits of this drug?
Intraoperative use of CYTALUX with a near infrared (NIR) imaging device helped surgeons remove additional cancer lesions that were not identified under normal light or palpation in patients with ovarian cancer undergoing debulking surgery.
What are the benefits of this drug (results of trials used to assess efficacy)?
The study assessed the proportion of patients with at least one evaluable ovarian cancer lesion confirmed by central pathology that was detected with CYTALUX under fluorescent light but not under normal light or palpation and not otherwise identified for resection prior to surgery. The detection proportion was estimated in females who underwent both normal light and fluorescent light (Intent-to-Image Set). The detection performance for the Intent-to-Image set met the pre-specified success threshold.
Table 1. Detection Proportion With CYTALUX Under Fluorescent Light but Not Under Normal Light or Palpation in the Intent-To-Image Set
| Assessment Statistic | Intent-To-Image Set N=134 |
|---|---|
| Patients with at least one confirmed ovarian cancer evaluable lesion | |
| Number (n) | 36 |
| Proportion (%) | 0.269 (26.9) |
| 95% CI (proportion)1 | 0.196*, 0.352 |
Source: FDA Statistical Reviewer’s Analysis Results
1 95% CIs are calculated using the exact Clopper-Pearson method
* The lower bound of the 95% confidence interval based on exact binomial exceeds the prespecified proportion of 0.10. Abbreviations: CI, confidence interval
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All trial participants were female; therefore, sex differences cannot be determined.
- Race: CYTALUX worked equally well in patients with ovarian cancer of all races studied in the clinical trial.
- Age: CYTALUX worked equally well in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2. Primary Endpoint by Subgroup Age
| Assessment Statistic | <65 Years N=87 |
≥65 Years N=47 |
|---|---|---|
| Patients with at least one confirmed ovarian cancer evaluable lesion | ||
| Number (n) | 24 | 12 |
| Proportion (%) | 0.276 (27.6) | 0.255 (25.5) |
| 95% CI (proportion)1 | 0.185, 0.382 | 0.140, 0.404 |
Source: FDA Statistical Reviewer’s Results
1 95% CIs are calculated using the exact Clopper-Pearson method
Abbreviations: CI, confidence interval
Table 3. Primary Endpoint by Subgroup Race
| Assessment Statistic | American Indian/ Alaska Native N=4 |
Asian N=5 |
Black or African American N=7 |
White N=114 |
Other N=4 |
|---|---|---|---|---|---|
| Patients with at least one confirmed ovarian cancer evaluable lesion | |||||
| Number (n) | 1 | 1 | 1 | 32 | 1 |
| Proportion (%) | 0.25 (25) | 0.2 (20) | 0.143 (14.3) | 0.281 (28.1) | 0.25 (25) |
| 95% CI (proportion)1 | 0.006, 0.806 | 0.005, 0.716 | 0.004, 0.579 | 0.201, 0.373 | 0.006, 0.806 |
Source: FDA Statistical Reviewer’s Results
1 95% CIs are calculated using the exact Clopper-Pearson method
Abbreviations: CI, confidence interval
What are the possible side effects?
The most common side effects of CYTALUX are nausea, vomiting, abdominal pain, flushing, dyspepsia, chest discomfort, and pruritus with 2.4% of patients experiencing reactions during the period of administration of CYTALUX. Reactions typically occurred within 15 minutes of the start of infusion. Treatment with antihistamines and/or anti-nausea medication may be used. If an adverse reaction occurs during administration, the infusion can be interrupted and resumed after treatment of the reaction.
What are the possible side effects (results of trials used to assess safety)?
Adverse reactions that occurred in >1% of patients were nausea (15%), vomiting (5.8%), abdominal pain (2.7%), flushing (1.7%), dyspepsia (1%), chest discomfort (1%), pruritus (1%), and hypersensitivity (1%). In 2.4% of patients, these adverse reactions occurred during the administration of CYTALUX.
Use of folate, folic acid, or folate-containing supplements may reduce binding of CYTALUX to folate receptors overexpressed on ovarian cancer cells and could reduce the detection of malignant lesions with CYTALUX. Avoid administration of folate, folic acid, or folate-containing supplements within 48 hours before administration of CYTALUX.
Were there any differences in side effects among sex, race and age?
- Sex: All trial participants were female; therefore, sex differences cannot be determined.
- Race: The occurrence of side effects was similar across all races studied in the clinical trials
- Age: The occurrence of side effects between patients younger and older than 65 years of age was similar.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
| All Patients | All Grades | Grades 3 or Higher | |
|---|---|---|---|
| Characteristic | Cytalux N=294 |
Cytalux N=294 |
Cytalux N=294 |
| Sex, n (%) | |||
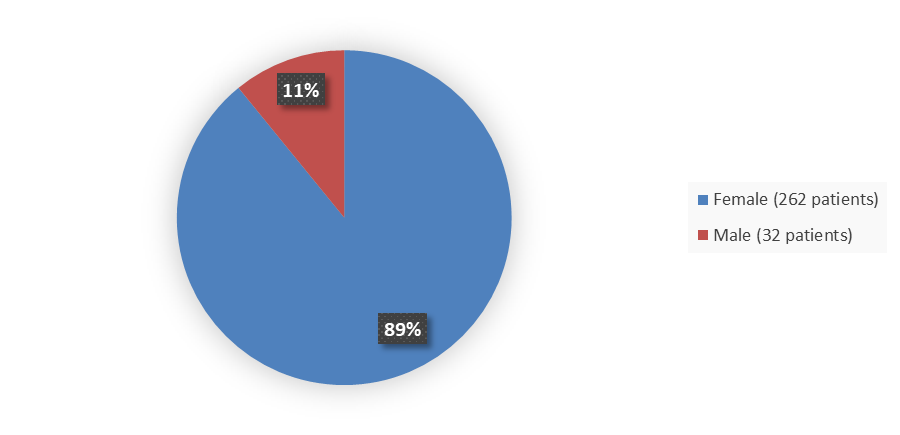
| Female | 262 (89.1) | 67/262 (25.6) | 22/262 (8.4) |
| Male | 32 (10.9) | 29/32 (90.6) | 9/32 (28.1) |
| Race, n (%) | |||
| American Indian or Alaska Native | 5 (1.7) | 1/5 (20.0) | 0/5 (0) |
| Asian | 17 (5.8) | 7/17 (41.2) | 2/17 (11.8) |
| Black or African American | 14 (4.8) | 6/14 (42.9) | 3/14 (21.4) |
| Multiple | 1 (0.3) | 0/1 (0) | 0/1 (0) |
| Other | 8 (2.7) | 0/8 (0) | 0/8 (0) |
| White | 248 (84.4) | 82/248 (33.1) | 26/248 (10.5) |
| Missing | 1 (0.3) | 0/1 (0) | 0/1 (0) |
| Age group, years, n (%) | |||
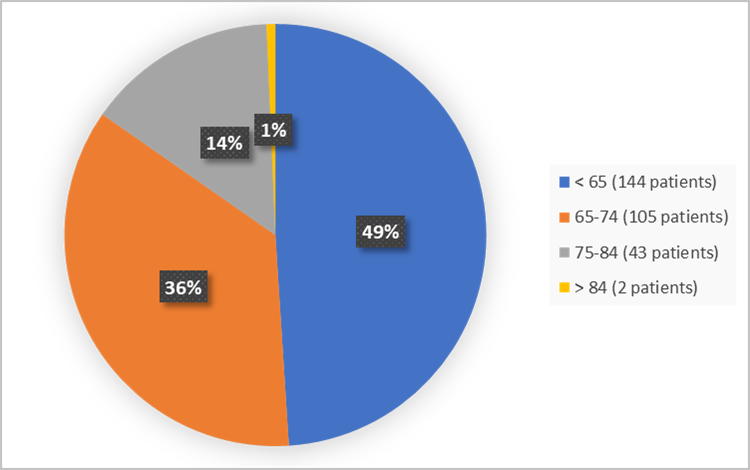
| < 65 | 144 (49.0) | 29/144 (20.1) | 8/144 (5.6) |
| > 84 | 2 (0.7) | 1/2 (50.0) | 1/2 (50.0) |
| 65-74 | 105 (35.7) | 46/105 (43.8) | 15/105 (14.3) |
| 75-84 | 43 (14.6) | 20/43 (46.5) | 7/43 (16.3) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 19 (6.5) | 2/19 (10.5) | 1/19 (5.3) |
| Not Hispanic or Latino | 264 (89.8) | 93/264 (35.2) | 30/264 (11.4) |
| Not Reported | 1 (0.3) | 0/1 (0) | 0/1 (0) |
| Unknown | 10 (3.4) | 1/10 (10.0) | 0/10 (0) |
Source: adsl.xpt and adae.xpt; Software: R
Analyses performed using safety population, defined as subjects who were randomized and started treatment.
DEMOGRAPHICS SNAPSHOT
Safety of CYTALUX was assessed in two ovarian cancer trials and one lung cancer trial.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
Figure 2 below summarizes how many patients by sex were in the combined ovarian and lung cancer trials used to evaluate the side effects of CYTALUX.
Figure 2. Baseline Demographics by Sex (Safety Population)
Source: Adapted from FDA Review
Figure 3 summarizes the percentage of patients by race enrolled in the combined clinical trials used to evaluate the efficacy of CYTALUX.
Figure 3. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
Figure 4. Baseline Demographics by Age for the Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 4. Demographics of Efficacy and Safety Trials by Race (Efficacy and Safety Populations)
| Race | Efficacy - Ovarian Cancer Study 006 | Safety - Ovarian and Lung Cancer |
|---|---|---|
| White | 127 | 248 |
| Asian | 7 | 17 |
| American Indian or Alaska Native | 4 | 5 |
| Black or African American | 7 | 7 |
| Others | 5 | 10 |
| Total | 150 | 294 |
Source: Adapted from FDA Review
How were the trials designed?
CYTALUX was evaluated in a clinical trial of population of 150 patients with ovarian cancer.
The primary efficacy endpoint in Study OTL-006, the primary study, was the proportion of patients with at least one ovarian cancer lesion confirmed by histopathology (standard of truth) that was detected using CYTALUX plus NIR fluorescent light but not under normal light or palpation.
How were the trials designed?
A total of 178 patients were enrolled for participation in Study OTL-006. Of these, 28 patients did not receive CYTALUX resulting in a total of 150 receiving drug and were included in the Safety population. From the 150 patients receiving CYTALUX, 134 patients were randomly assigned to the fluorescent and normal light group, 6 patients to normal light only, and 10 patients were not randomly assigned to an imaging group.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.