Drug Trials Snapshot: AVYCAZ (cIAI)
For the treatment of complicated intra-abdominal infections
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that support the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the AVYCAZ Prescribing Information
AVYCAZ (ceftazidime-avibactam)
(Av-EE-Kaz)
Forest Pharmaceuticals, Inc.
Approval date: February 25, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
AVYCAZ is a drug used to treat adults who have a serious infection in their belly called a complicated intra-abdominal infection (abbreviated as cIAI). It should be used only when there are few or no other treatment options. AVYCAZ is also approved for patients with a serious infection in their urinary tract system called a complicated urinary tract infection. This is discussed in a separate Snapshot.
AVYCAZ is a combination of two drugs: ceftazidime, a previously-approved drug that fights bacteria (antibiotic) and avibactam, a new drug that improves how ceftazidime works.
AVYCAZ is intended to be used along with another antibiotic, called metronidazole, for the treatment of cIAI.
How is this drug used?
AVYCAZ is a drug administered by a health care professional directly into the bloodstream though a needle in the vein. This is known as an intravenous, or IV, infusion.
What are the benefits of this drug?
In patients who have few or no other treatment options, AVYCAZ would be expected to help improve the symptoms of cIAI.
What are the benefits of this drug (results of trials used to assess efficacy)?
The determination of efficacy of AVYCAZ was supported in part by the previous findings of the efficacy of ceftazidime for the treatment of cIAI. The contribution of avibactam to AVYCAZ was primarily established in vitro and in animal models of infection. AVYCAZ was studied in a Phase 2, randomized, blinded, active-controlled, multicenter trial in adults with cIAI. The trial was not designed with any formal hypothesis for inferential testing against the active comparator.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The studies that looked at the benefits of AVYCAZ were too small to determine if there were differences in sex, race, and age subgroups.
There are currently limited data on the efficacy of AVYCAZ. Therefore, subgroup analysis for efficacy cannot be performed.
What are the possible side effects?
In patients treated for cIAI, the most common side effects of AVYCAZ were vomiting and nausea.
Severe allergic reactions can occur. Patients who are allergic to penicillin may also be allergic to AVYCAZ.
Serious side effects, such as seizures and coma, can occur more frequently in patients whose kidneys do not work as well as they should. These are known effects of the ceftazidime component of AVYCAZ.
What are the possible side effects (results of trials used to assess safety)?
Table 5 summarizes the most common adverse events observed in the trial.
Table 5. Incidence of Selected Adverse Reactions Occurring in 5% or More of Patients Receiving AVYCAZ in the Trial (Safety Population)
| Adverse Event | AVYCAZ + metronidazole N=101 | Meropenem N=102 |
|---|---|---|
| Vomiting | 14% | 5% |
| Nausea | 10% | 6% |
| Increased blood alkaline phosphatase | 9% | 7% |
| Abdominal pain | 8% | 3% |
| Increased alanine aminotranferase | 8% | 13% |
| Anxiety | 5% | 1% |
| Constipation | 4% | 1% |
| Upper abdominal pain | 1% | 0% |
| Dizziness | 0% | 2% |
Source: Extracted from AVYCAZ Package Insert, Section 6, Table 4
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of overall side effects was similar in men and women.
- Race: The number of patients in the non-White subgroups was limited. Therefore, differences among race could not be detected.
- Age: The number of patients 65 years of age and above was limited. Therefore, differences in response to AVYCAZ between patients above and below 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
Table 6 summarizes the incidence of treatment-emergent adverse events by subgroup.
Table 6. Summary of Incidence of Treatment-Emergent Adverse Events (TEAE) by Subgroups (Safety Population)
| Subgroup | AVYCAZ + Metronidazole (N=101) | Meropenem (N=102) |
|---|---|---|
| n/N (%) | n/N (%) | |
| Overall Response/All patients | 61/101 (60.4) | 59/102 (57.8) |
| Sex | ||
| Male | 42/70 (60.0) | 46/81 (56.8) |
| Female | 19/31 (61.3) | 13/21 (61.9) |
| Age Group | ||
| =17 - 65> | 56/94 (59.6) | 50/88 (56.8) |
| >=65 years | 5/7 (71.4) | 9/14 (64.3) |
| >=75 years | 1/2 (50.0) | 3/3 (100) |
| Race | ||
| White | 34/56 (60.7) | 36/65 (55.4) |
| Black or African American | 0/0 (0) | 1/1 (100.0) |
| Asian | 15/32 (46.9) | 9/23 (39.1) |
| American Indian or Alaska Native | 1/1 (100) | 0/0 (0) |
| Native Hawaiian or Other Pacific Islander | 0/0 (0) | 0/0 (0) |
| Other | 11/12 (91.7) | 13/13 (100) |
Source: From Clinical Reviewer
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
FDA approved AVYCAZ based on the prior approval and known benefit of ceftazidime as well as more recent evidence from the laboratory and from a clinical trial of 203 patients with cIAI. The trial included patients from the United States, Europe, and Asia.
Figure 1 summarizes how many patients by sex were enrolled in the clinical trial to evaluate safety.
Figure 1. Baseline Demographics by Sex (Safety)
Source: Extracted from Clinical Review, Table 50
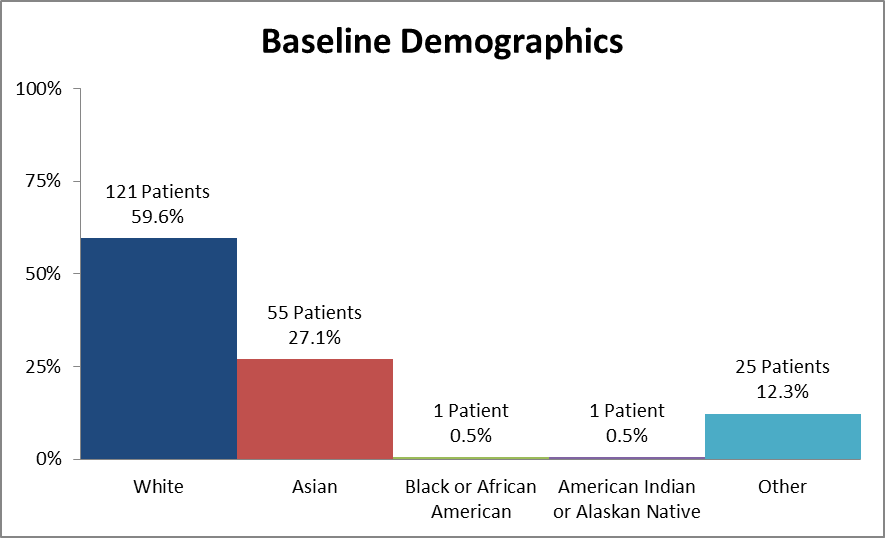
Figure 2 and Table 1 summarize the number of patients of each race who were enrolled in the clinical trial to assess safety.
Figure 2. Baseline Demographics by Race (Safety)
Source: Extracted from Clinical Review, Table 50
Table 2. Baseline Demographics by Race (Safety Population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 121 | 59.6% |
| Asian | 55 | 27.1% |
| Black or African American | 1 | 0.5% |
| American Indian or Alaskan Native | 1 | 0.5% |
| Other | 25 | 12.3% |
Source: Extracted from Clinical Review, Table 50
The figure below summarizes the number of patients in each age group who were enrolled in the clinical trial assessing safety.
Figure 3. Baseline Demographics by Age (Safety Population)
Source: From Clinical Reviewer
Table 8 summarizes baseline demographic information for the safety population.
Table 8. Baseline Demographics for the Trial (Safety Population)
| Demographic Parameter | AVYCAZ + Metronidazole (N=101) n (%) | Meropenem (N=102) n (%) |
|---|---|---|
| Sex | ||
| Male | 70 (69.3) | 81 (79.4) |
| Female | 31 (30.7) | 21 (20.6) |
| Age (years) | ||
| Mean (SD) | 43.2 (16.0) | 42.9 (18.1) |
| Median | 41 | 39 |
| Min, Max | 18, 80 | 19, 88 |
| Age Group (years) | ||
| 17 to 64 | 92 (91.1) | 87 (85.3) |
| >=65 | 9 (8.9) | 15 (14.7) |
| >=75 | 2 (2.0) | 3 (2.9) |
| Race | ||
| White | 56 (55.4) | 65 (63.7) |
| Black or African American | 0 (0.0) | 1 (1.0) |
| Asian | 32 (31.7) | 23 (22.5) |
| American Indian or Alaskan Native | 1 (1.0) | 0 (0.0) |
| Other | 12 (11.9) | 13 (12.7) |
| Ethnicity | ||
| Hispanic or Latino | 3 (3.0) | 2 (2.0) |
| Not Hispanic or Latino | 98 (97.0) | 100 (98.0) |
| Region | ||
| United States | 12 (11.9) | 7 (6.9) |
| Europe | 42 (41.6) | 58 (56.9) |
| Asia | 47 (46.5) | 37 (36.3) |
Source: From Clinical Reviewer
How were the trials designed?
In the clinical trial, half of the patients were chosen at random to receive AVYCAZ along with a drug called metronidazole. The other half were given a different antibiotic called meropenem. Neither the patients nor the health care professionals administering the drug knew which patients were taking AVYCAZ plus metronidazole and which were taking only meropenem until after the study was complete.
How were the trials designed?
Patients were randomly assigned to receive AVYCAZ plus metronidazole or meropenem. Patients were stratified by baseline severity of disease (APACHE II score 10,="" and=""> 10 to ≤ 25). Neither the patients nor the health care professional administering the drug knew which patients were in which group until after the trial was complete. Treatment duration was 5 to 14 days.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION
MEDICAL REVIEW