Drug Trials Snapshot: LIVMARLI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the LIVMARLI Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
LIVMARLI (maralixibat chloride)
(liv mar' lee)
Mirum Pharmaceuticals, Inc.
Original Approval date: September 29, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LIVMARLI is a drug for the treatment of itching (pruritus) due to blockage of bile flow out of the liver (cholestasis) in patients with Alagille syndrome (ALGS) one year of age and older.
ALGS is an inherited disorder than can affect the liver and other parts of the body.
How is this drug used?
LIVMARLI is an oral solution taken by mouth daily. The dose of LIVMARLI is based on the patient’s weight.
Who participated in the clinical trials?
The FDA approved LIVMARLI based on evidence of efficacy from a clinical trial (Trial 1/NCT02160782) of 29 randomized patients with ALGS with cholestasis and at least moderate pruritus. The trial was conducted at 9 sites in 6 countries (Australia, Belgium, France, Poland, Spain, and the United Kingdom).
In addition to the trial used to evaluate the efficacy of LIVMARLI, there were two additional trials that were used to evaluate the safety of LIVMARLI. These trials were conducted in the United States, Canada, and the United Kingdom.
The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
What are the benefits of this drug?
In the trial, patients with ALGS who had moderate to severe itching were given LIVMARLI. After 18 weeks of LIVMARLI treatment, patients were randomly assigned to switch to placebo or continue LIVMARLI. Patients who continued LIVMARLI had less itching than those who switched to placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
In the trial, 29 ALGS patients with moderate to severe pruritus received LIVMARLI for 18 weeks. Patients were then randomized to either remain on LIVMARLI treatment or withdrawn to placebo for 4 weeks through Week 22. Given the patients’ young age, caregivers assessed patients’ scratching twice daily (once in the morning and once in the evening), on a scale ranging from 0 (none observed or reported) to 4 (very severe).
Table 1. Weekly Average of Worst Daily Scratching Severity Scores1,2
|
|
LIVMARLI |
Placebo |
Mean Difference |
|---|---|---|---|
|
Mean at Week 22 (95% CI) |
1.6 (1.1, 2.1) |
3.0 (2.6, 3.5) |
-1.4 (-2.1, -0.8) |
|
Mean change from Week 183 to Week 22 (95% CI) |
0.2 (-0.3, 0.7) |
1.6 (1.2, 2.1) |
Source: LIVMARLI Prescribing information
1 Weekly average scores for each patient were calculated using the worst score per day (morning or evening), and the average was then calculated for each treatment group. Higher scores and larger increases from Week 18 represent worse itching.
2 Results based on an analysis of covariance model with treatment group and Week 18 average worst daily pruritus score as covariates.
3 The mean (SD) at baseline (pre-treatment) was 3.1 (0.5) and the mean (SD) at Week 18 (pre-randomized withdrawal period) was 1.4 (0.9).
Abbreviations: CI, confidence interval; SD, standard deviation
After re-entering the open-label treatment phase after Week 22, both randomized treatment groups had similar mean pruritus scores by Week 28, the first week placebo patients received the full dosage of LIVMARLI after withdrawal.
Figure 1. Weekly Average of Worst Daily Scratching Severity Scores1 (Observed Data)
Source: LIVMARLI Prescribing Information
1 Weekly average scores for each patient were calculated using the worst score per day (morning or evening), and the average was then calculated for each treatment group. Higher scores and larger increases from Week 18 represent worse itching. RW Period = Randomized Withdrawal period (Week 19 to Week 22) where patients were randomized to remain on LIVMARLI or withdrawn from treatment to receive placebo. Prior to and after the RW Period, all patients received LIVMARLI.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Race: Data not collected.
- Sex and Age: The trial was too small to determine if there were any differences in sex and age subgroups.
What are the possible side effects?
LIVMARLI may cause serious adverse reactions, including changes in liver tests, abdominal pain, diarrhea, and vitamin deficiencies, such as vitamin A, D, E, and K. Because of possible vitamin K deficiency, there may be a risk of bleeding if taken with anticoagulants.
Other common side effects reported during treatment with LIVMARLI are bone fractures and gastrointestinal bleeding.
What are the possible side effects (results of trials used to assess safety)?
Table 2. Adverse Reactions Reported in ≥5% of Patients With ALGS in LIVMARLI Clinical Studies
|
Adverse Reaction |
LIVMARLI |
|---|---|
|
Diarrhea |
48 (56) |
|
Abdominal pain |
46 (53) |
|
Vomiting |
35 (41) |
|
Nausea |
7 (8) |
|
Fat-soluble vitamin deficiency |
22 (26) |
|
Transaminases increased (ALT, AST) |
16 (19) |
|
Gastrointestinal bleeding |
9 (10) |
|
Bone fractures |
8 (9) |
Source: LIVMARLI Prescribing Information
Abdominal pain includes: abdominal discomfort or pain
Fat-soluble vitamin deficiency includes: A, D, E, or K deficiency, or INR increase
Abbreviations: ALGS, Alagille syndrome; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio
Were there any differences in side effects of the clinical trials among sex, race, and age?
The trials were too small to determine if there were any differences in sex, race, and age subgroups.
DEMOGRAPHICS SNAPSHOT
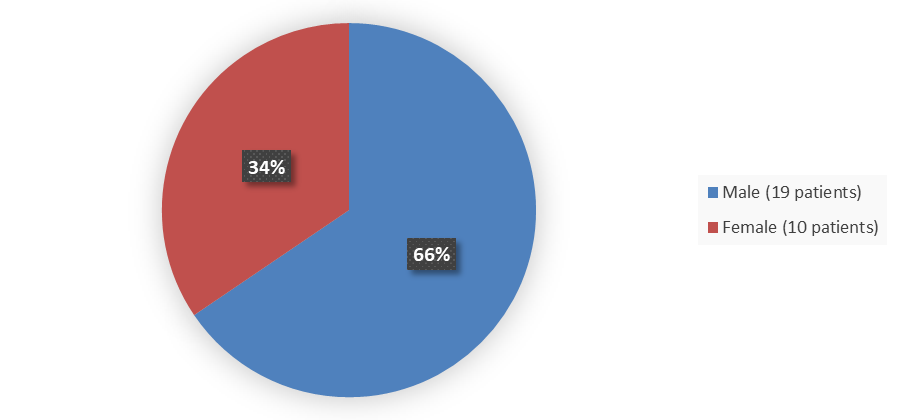
Figure 2 summarizes how many patients by sex were enrolled in the clinical trial used to evaluate the efficacy of LIVMARLI.
Figure 2. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
Figure 3 below summarizes how many patients by sex were in the combined trials used to evaluate the side effects of LIVMARLI.
Figure 3. Baseline Demographics by Sex (Safety Population)
Source: Adapted from FDA Review
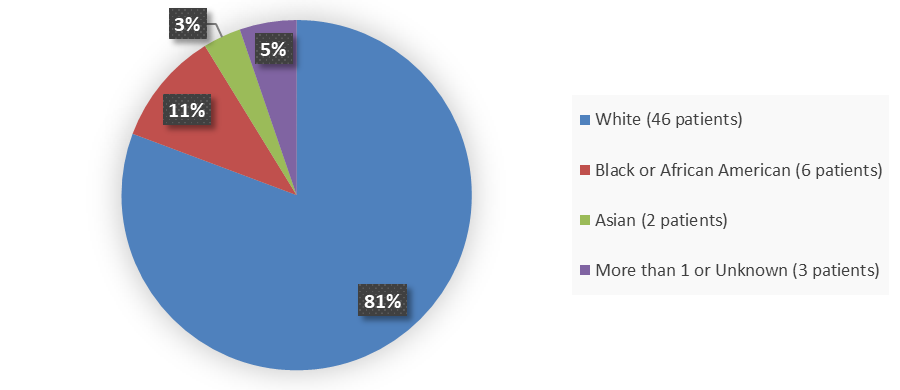
Data on race was not collected in the trial used to evaluate the efficacy of LIVMARLI (Trial 1) but was collected in the two additional trials that were also used to evaluate the safety of LIVMARLI.
Figure 4 below summarizes how many patients by race were in the combined trials used to evaluate the side effects of LIVMARLI, excluding patients from Trial 1 because lack of collection of race data.
Figure 4. Baseline Demographics by Race (Safety Population Excluding Patients From Trial 1)
Source: Adapted from FDA Review
Figure 5 below summarizes how many patients by age were in the trial used to evaluate the efficacy of LIVMARLI.
Figure 5. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
Figure 6 below summarizes how many patients by age were in the combined trials used to evaluate the side effects of LIVMARLI.
Figure 6. Baseline Demographics by Age (Safety Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 3. Baseline Demographics of Enrolled Patients in the Clinical Trial (Efficacy Population)
|
Demographic Parameters |
Trial 1 |
|---|---|
|
Sex, n (%) |
|
|
Male |
19 (66) |
|
Female |
10 (34) |
|
Age, years |
|
|
Mean (SD) |
5.6 (4.3) |
|
Median |
5 |
|
Min, max |
1, 15 |
|
Age group, years, n (%) |
|
|
<2 |
5 (17) |
|
2 to 4 |
8 (28) |
|
5 to 8 |
9 (31) |
|
9 to 12 |
4 (14) |
|
13 to 18 |
3 (10) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
How were the trials designed?
There was one trial that evaluated the efficacy of LIVMARLI in 29 ALGS patients with moderate to severe pruritus (itching). Patients treated with LIVMARLI for 22 weeks were compared to patients who were treated with LIVMARLI for 18 weeks and then switched to placebo treatment. Given the patients’ young age, caregivers assessed patients’ scratching twice daily (once in the morning and once in the evening) on a scale ranging from 0 (none observed or reported) to 4 (very severe).
How were the trials designed?
The efficacy of LIVMARLI was evaluated in a single study which included an 18-week open-label treatment period followed by a 4-week randomized, double-blind, placebo-controlled drug-withdrawal period.
Given the patients’ young age, caregivers assessed patients’ scratching twice daily (once in the morning and once in the evening) on a scale ranging from 0 (none observed or reported) to 4 (very severe). Weekly average scores for each patient were calculated using the worst score per day (morning or evening), and the average was then calculated for each treatment group for comparison.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.