Drug Trials Snapshot: LIVTENCITY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the LIVTENCITY Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
LIVTENCITY (maribavir)
(liv ten’ si tee)
Takeda Pharmaceuticals USA, Inc.
Original Approval date: 11/23/2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LIVTENCITY is a prescription medicine used to treat cytomegalovirus (CMV) infection and disease in adults and children 12 years of age and older and weighing at least 77 pounds (35 kg) who have received a solid organ or stem cell transplant, when their infection or disease does not respond to treatment with ganciclovir, valganciclovir, cidofovir, or foscarnet.
How is this drug used?
LIVTENCITY is a prescription medicine taken by mouth in the form of a tablet two times a day.
Who participated in the clinical trials?
The FDA approved LIVTENCITY based on evidence from one clinical trial of 352 transplant patients who had CMV infection or disease not responding to treatment with ganciclovir, valganciclovir, cidofovir, or foscarnet. The trial was conducted at 94 sites in North America (United States and Canada), Europe (Austria, Belgium, Croatia, Denmark, France, Germany, Italy, Spain, Switzerland, and the United Kingdom), and Asia Pacific (Australia and Singapore).
What are the benefits of this drug?
In the trial, LIVTENCITY was compared with investigator’s assigned treatment which included one or two of the following prescription medicines: ganciclovir, valganciclovir, foscarnet, or cidofovir. The trial compared the two groups’ plasma CMV DNA concentration levels at the end of the study’s eighth week. Of the patients who took LIVTENCITY, 56% had CMV DNA levels below what was measurable versus 24% of patients who received the investigator-assigned treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 below summarizes efficacy results from the clinical trial in transplant patients who had CMV infection or disease not responding to treatment with ganciclovir, valganciclovir, cidofovir, or foscarnet. The primary endpoint was the proportion of subjects who had no measurable CMV DNA concentration levels at the end of the study’s eighth week.
Table 1. Primary Efficacy Endpoint Analysis at Week 8 (Randomized Set)
| Primary Endpoint: Confirmed CMV DNA Level <LLOQ at Week 8a | LIVTENCITY 400 mg BID | IAT |
|---|---|---|
| Responders | 131 (56) | 28 (24) |
| Adjusted difference in proportion of responders (95% CI)b | 33 (23, 43) | |
| p-value, adjustedb | <0.001 | |
Source: LIVTENCITY Prescribing Information
a Confirmed CMV DNA level <LLOQ at the end of 8 weeks of treatment (2 consecutive samples separated by at least 5 days with DNA levels <LLOQ, i.e., <137 IU/mL).
b Cochran-Mantel-Haenszel weighted average approach was used for the adjusted difference in proportion (LIVTENCITY – IAT), the corresponding 95% CI, and the p-value after adjusting for the transplant type and baseline plasma CMV DNA concentration. Only those with both stratification factors were included in the computation.
Abbreviations: BID, twice daily; CI, confidence interval; CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplant; IAT, investigator assigned anti CMV treatment; LLOQ, lower limit of quantification; N, number of patients; SOT, solid organ transplant.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: LIVTENCITY worked similarly in males and females.
- Race: LIVTENCITY worked similarly in White and Black or African American patients.
- Age: LIVTENCITY worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 below summarizes the efficacy results based on sex, race, and age.
Table 2. Subgroup Analyses Based on Sex, Race, and Age
| Demographic Characteristics | LIVTENCITY | IAT |
|---|---|---|
| Sex | ||
| Race | ||
| Age | ||
| Female | 44/87 (51) | 13/52 (25) |
| Male | 87/148 (59) | 15/65 (23) |
| Black or African American | 17/29 (59) | 8/18 (44) |
| White | 100/179 (56) | 18/87 (21) |
| Asian | 5/9 (56) | 1/7 (14) |
| Other | 7/16 (44) | 1/5 (20) |
| 18 to 44 years | 28/55 (51) | 8/32 (25) |
| 45 to 64 years | 71/126 (56) | 19/69 ((28) |
| ≥ 65 years | 32/54 (59) | 1/16 (6) |
Source: Adapted from FDA Review
Abbreviations: IAT, investigator assigned treatment
What are the possible side effects?
The most common side effects of LIVTENCITY include dysgeusia (change in taste), nausea, diarrhea, vomiting, and tiredness.
What are the possible side effects (results of trials used to assess safety)?
Table 3 is a summary of the most common adverse events with a frequency of more than 10% of subjects in the LIVTENCITY group that were observed in the controlled trial.
Table 3. Adverse Events Occurring in >10% of Subjects Receiving LIVTENCITY in the Controlled Trial
Adverse Event | LIVTENCITY | IATa |
|---|---|---|
| Taste disturbanceb | 46 | 4 |
| Nausea | 21 | 22 |
| Diarrhea | 19 | 21 |
| Vomiting | 14 | 16 |
| Fatigue | 12 | 9 |
Source: LIVTENCITY Prescribing information
a IAT included monotherapy or dual therapy with ganciclovir, valganciclovir, foscarnet, or cidofovir as dosed by the investigator
b Taste disturbance includes the following reported preferred terms: ageusia, dysgeusia, hypogeusia and taste disorder Abbreviations: IAT, investigator-assigned treatment
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar in White and Black or African American patients.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 4 below summarizes the frequency of treatment-emergent adverse events (observed during the on-treatment period) based on sex, race, and age. The number of patients in races other than White and Black or African American was limited. Therefore, differences in treatment-emergent adverse events in these races (Asian, other) could not be determined.
Table 4. Treatment-Emergent Adverse Events Based on Sex, Race, and Age (During On-Treatment Observation Period)
Demographic Characteristics | Treatment-Emergent Adverse Events | |
|---|---|---|
LIVTENCITY | IAT | |
| Sex | ||
| Race | ||
| Age, years | ||
| Female | 99 | 92 |
| Male | 97 | 91 |
| Black or African American | 93 | 94 |
| White | 98 | 89 |
| 18 to 44 | 96 | 91 |
| 45 to 64 | 97 | 90 |
| ≥65 | 100 | 100 |
Source: Adapted from FDA review
Abbreviations: IAT, investigator-assigned treatment
DEMOGRAPHICS SNAPSHOT
Figure 1 summarizes how many male and female patients were enrolled in the controlled clinical trial to evaluate the efficacy of LIVTENCITY.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
Figure 2 summarizes the percentage of patients by race enrolled in the controlled clinical trial used to evaluate the efficacy of LIVTENCITY.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
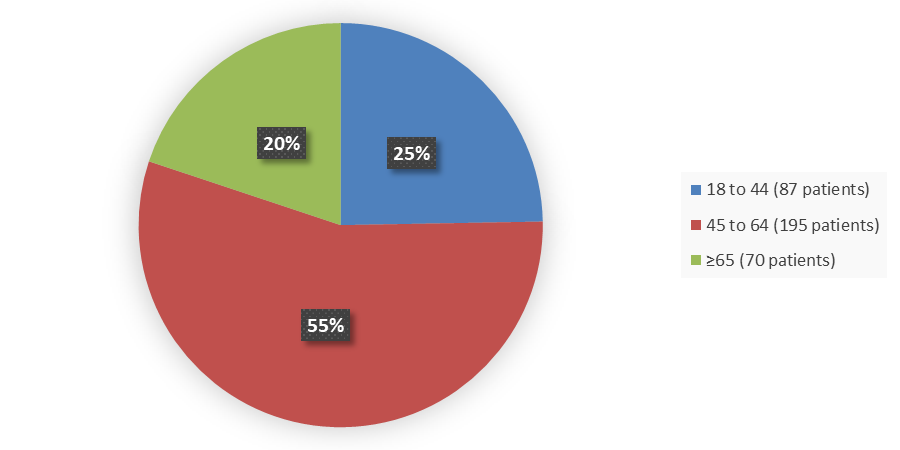
Figure 3 summarizes the percentage of patients by age in the controlled clinical trial.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
Who participated in the trials?
Table 5. Demographics of Trial by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 266 | 76 |
| Black or African American | 47 | 13 |
| Asian | 16 | 5 |
| Other | 21 | 6 |
| Missing | 2 | <1 |
Source: Adapted from FDA Review
How were the trials designed?
FDA approved LEVTENCITY based on evidence from one clinical trial of 352 patients who received a transplant and had cytomegalovirus infection which was not responding to treatment with ganciclovir, valganciclovir, cidofovir, or foscarnet. Patients were randomized to receive either LIVTENCITY or treatment assigned by a researcher running the study for up to eight weeks. The trial compared plasma CMV DNA concentration levels between the two groups at the end of the study’s eighth week.
How were the trials designed?
The evaluation of LIVTENCITY was primarily based on one multicenter, randomized, open-label, active-controlled trial designed to assess the safety and efficacy of LIVTENCITY compared to Investigator-Assigned Treatment (IAT) for the treatment of post-transplant CMV infections in sold organ or hematopoietic transplant recipients which were refractory to treatment (with or without genotypic resistance) with ganciclovir, valganciclovir, cidofovir, or foscarnet. A total of 352 patients were randomized in a 2:1 ratio to receive either LIVTENCITY 400 mg twice daily or IAT for 8 weeks. Upon completion of the treatment period, enrolled subjects entered a 12-week follow-up period.
The primary efficacy outcome was the proportion of subjects with confirmed CMV viremia “clearance” defined as unquantifiable plasma CMV DNA (less than the lower limit of quantification, i.e., <137 IU/mL) at the end of Week 8.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
LINK TO DRUG PACKAGE INSERT