Drug Trials Snapshot: LUPKYNIS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the LUPKYNIS Package Insert for complete information.
LUPKYNIS (voclosporin)
(loop kye’ nis)
Aurinia Pharmaceuticals
Approval date: January 22, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LUPKYNIS is a drug used to treat patients with active lupus nephritis.
Lupus nephritis (LN) is an inflammation of the kidneys that is caused by systemic lupus erythematous (SLE) - a chronic, autoimmune disease.
How is this drug used?
LUPKYNIS is a capsule that is taken twice daily by mouth in combination with two other drugs (mycophenolate mofetil and corticosteroid).
What are the benefits of this drug?
In the clinical trial, a greater proportion of patients who received LUPKYNIS for one year, achieved a reduction in the kidney inflammation in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results for Trial 1. The primary endpoint was complete renal response at Week 52.
Table 1. Complete Renal Response at Week 52 in Trial 1.
|
|
LUPKYNIS |
Placebo |
Odds Ratio |
|---|---|---|---|
|
Primary Endpoint |
|||
|
Complete Renal Response at Week 52 [n (%)]a |
73 (40.8) |
40 (22.5) |
2.7 (95% CI: 1.6, 4.3); |
|
Components of Primary Endpoint |
|||
|
UPCR ≤ 0.5 mg/mg [n (%)] |
81 (45.3) |
41 (23.0) |
3.1 (95% CI: 1.9, 5.0) |
|
eGFR ≥ 60 mL/min/1.73 m2 or no confirmed decrease |
147 (82.1) |
135 (75.8) |
1.5 (95% CI: 0.8, 2.5) |
eGFR = Estimated glomerular filtration rate, uPCR: Urine protein-creatinine ratio CI: Confidence interval,
- In order to be considered a responder, the patient must not have received more than 10 mg prednisone for ≥ 3 consecutive days or for ≥ 7 days in total during Weeks 44 through 52. Patients who received rescue medication or withdrew from the study were considered non-responders.
- Treatment- or disease-related eGFR-associated event is defined as blood creatinine increased, creatinine renal clearance decreased, glomerular filtration rate decreased, serum creatinine increased, renal impairment, renal failure, or renal failure acute.
LUPKYNIS Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: LUPKYNIS worked similarly in men and women.
- Race: LUPKYNIS worked similarly in White and Asian patients.
- Age: The majority of patients were younger than 65 years of age, therefore the differences in how well LUPKYNIS worked between younger and older patients could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarize subgroup results based on the primary endpoint analyses of LUPKYNIS compared with placebo in Trial 1.
Table 2. Effects of LUPKYNIS on Adjudicated Renal Response at Week 52, Stratified by Sex, Race, and Age Subgroups in Trial 1
|
Demographic Parameter |
Response Rate % |
Odds Ratio a |
|
|---|---|---|---|
|
|
LUPKYNIS (N=179) |
Placebo (N=178) |
(95% CI) |
|
Sex |
|||
|
Men |
44.4% |
19.2 % |
4.1 (3.2, 5.1) |
|
Women |
40.4% |
23.0 % |
2.5 (2.2, 2.7) |
|
Race |
|||
|
White |
38.2 % |
29.5 % |
1.7 (1.3, 2.1) |
|
Asian |
41.5 % |
17.9 % |
4.0 (3.6, 4.5) |
|
Other |
43.1 % |
19.7 % |
3.7 (3.3, 4.2) |
|
Age Group (years) |
|||
|
30 or younger |
40.4 |
18.1 |
3.2 (2.1, 4.4) |
|
Greater than 30 |
41.1 |
26.3 |
2.7 (2.3, 3.0) |
a= Odds ratios and credible intervals include relevance of outcomes from other subgroups. In order to be considered a responder, the patient must not have received more than 10 mg prednisone for ≥ 3 consecutive days or for ≥ 7 days in total during Weeks 44 through 52. Patients who received rescue medication or withdrew from the study were considered non-responders.
Adapted from FDA Statistical Review
What are the possible side effects?
LUPKYNIS may cause serious side effects including increased risk of developing cancer, infection, kidney toxicity, high blood pressure, nervous system problems, high potassium, heart rhythm problems due to prolongation of heart electrical activity (QT prolongation), and low red blood cell count (anemia).
The most common side effects are kidney toxicity, high blood pressure, diarrhea, headache, anemia, cough, and urinary tract infection.
What are the possible side effects (results of trials used to assess safety)?
Below is the summary of the most common adverse reactions observed in patients treated with LUPKYNIS at the recommended dose or placebo in combined Trials 1 and 2.
Table 3. Adverse Reactions in ≥3% of Patients Treated with LUPKYNIS 23.7 mg Twice a Day and ≥2% Higher than Placebo in Trials 1 and 2
|
Adverse Reaction
|
LUPKYNIS |
Placebo |
|---|---|---|
|
Glomerular filtration rate decreased |
26% |
9% |
|
Hypertension |
19% |
9% |
|
Diarrhea |
19% |
13% |
|
Headache |
15% |
8% |
|
Anemia |
12% |
6% |
|
Cough |
11% |
2% |
|
Urinary tract infection |
10% |
6% |
|
Abdominal pain upper |
7% |
2% |
|
Dyspepsia |
6% |
3% |
|
Alopecia |
6% |
3% |
|
Renal Impairment |
6% |
3% |
|
Abdominal pain |
5% |
2% |
|
Mouth ulceration |
4% |
1% |
|
Fatigue |
4% |
1% |
|
Tremor |
3% |
1% |
|
Acute kidney injury |
3% |
1% |
|
Decreased appetite |
3% |
1% |
LUPKYNIS Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The majority of patients were women. The differences in side effects between men and women were not analyzed.
- Race: The occurrence of side effects was similar across racial groups.
- Age: The majority of patients were younger than 65 years of age, therefore the differences in side effects between younger and older patients could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of adverse events by race subgroups. Sex and age subgroups were not analyzed because the majority of patients were women and younger than 65 years of age.
Table 4. Subgroup Analysis of Treatment-Emergent Adverse Events (TEAE) by Race
|
Adverse Event |
White |
Asian |
Black or African American |
Other |
||||
|---|---|---|---|---|---|---|---|---|
|
LUPKYNIS |
Placebo |
LUPKYNIS |
Placebo |
LUPKYNIS |
Placebo |
LUPKYNIS |
Placebo |
|
|
Any TEAE, n/N (%) |
83/98 (84.7) |
79/103 (76.7) |
101/105 (96.2) |
89/92 (96.7) |
29/29 (100) |
22/24 (91.7) |
31/35 (88.6) |
42/47 (89.4) |
|
Any Serious TEAE n/N (%) |
18/98 (18.4) |
22/103 (21.4) |
31/105 (29.5) |
15/92 (16.3) |
7/29 (24.1) |
8/24 (33.3) |
5/35 (14.3) |
5/47 (10.6) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved LUPKYNIS based on evidence from two clinical trials (Trial 1/(NCT03021499), and Trial 2 /NCT02141672) of 533 patients with lupus nephritis. The trials were conducted at 221 sites in 47 countries including the United States.
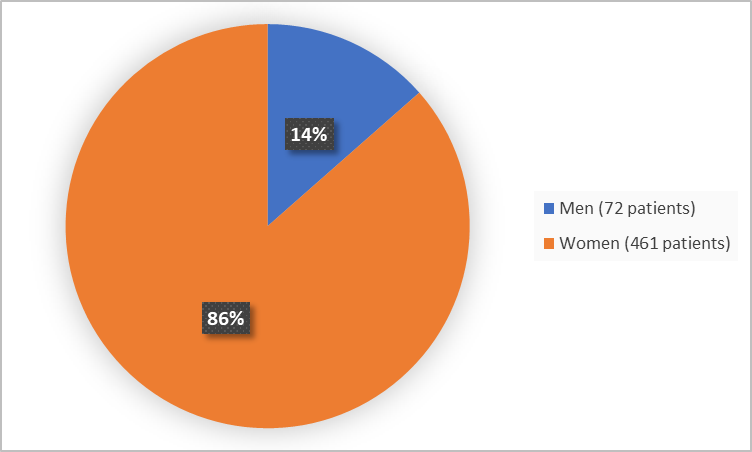
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex
Adapted from FDA Review
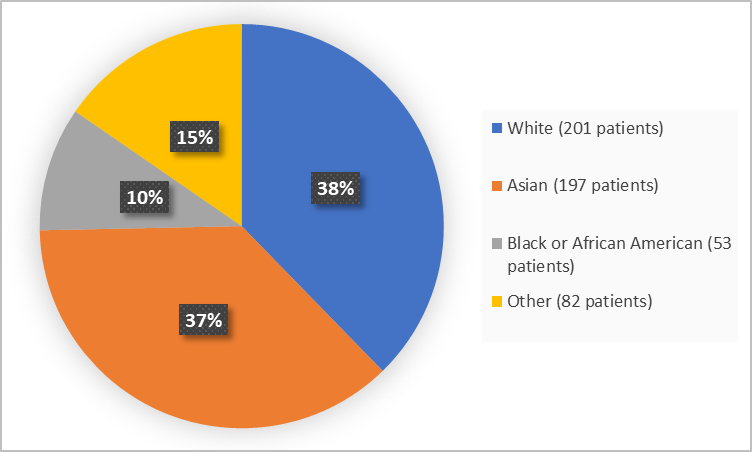
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Demographics by Race
Adapted from FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Demographics by Age
Adapted from FDA Review
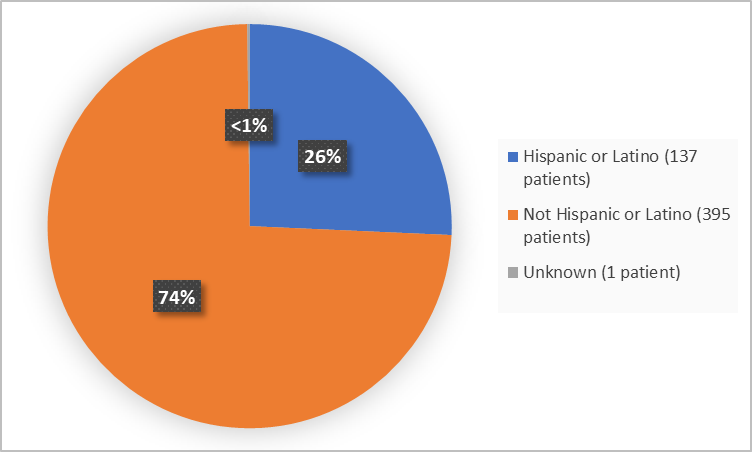
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials.
Figure 4. Demographics by Ethnicity
Adapted from FDA Review
Who participated in the trials?
The table below summarize demographics of patients in pooled Trials 1 and 2.
Table 5. Trial Demographics (safety population)
|
LUPKYNIS |
Placebo |
TOTAL |
|
|---|---|---|---|
|
Sex, n (%) |
|||
|
Women |
236 (88.4) |
225 (84.6) |
461 (86.5) |
|
Men |
31 (11.6) |
41 (15.4) |
72 (13.5) |
|
Race, n (%) |
|||
|
Asian |
105 (39.3) |
92 (34.6) |
197 (37.0) |
|
Black or African American |
29 (10.9) |
24 (9.0) |
53 (9.9) |
|
White |
98 (36.7) |
103 (38.7) |
201 (37.7) |
|
Other |
35 (13.1) |
47 (17.7) |
82 (15.4) |
|
Age, Median (Min, Max) |
30 (18,66) |
32 (18,72) |
31 (18,72) |
|
Age Group, n (%) |
|||
|
18-65 years |
266 (99.6) |
264 (99.2) |
530 (99.4) |
|
>65 years |
1 (0.4) |
2 (0.8) |
3 (0.6) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
65 (24.3) |
72 (27,1) |
137 (25.7) |
|
Not Hispanic or Latino |
202 (75.7) |
193 (72.6) |
395 (25.7) |
|
Unknown |
0 |
1 (0.4) |
1 (0.2) |
|
Geographic Region, n (%) |
|||
|
Asia |
104 (38.9) |
87 (32.7) |
191 (35.9) |
|
Europe + South Africa |
77 (28.8) |
86 (32.3) |
163 30.6) |
|
Americas |
86 (32.3) |
93 (35) |
179 (33.6) |
|
United States |
31 (11.6) |
32 (12) |
63 (11.8) |
Adapted from FDA Review
How were the trials designed?
There were two trials that provided data for LUPKYNIS approval. Trials enrolled patients with lupus nephritis.
In Trial 1, patients were randomly assigned to receive by mouth LUPKYNIS or placebo for 52 weeks. Neither the patient nor the healthcare providers knew which medication was being given. The benefit of LUPKINYS in comparison to placebo was assess by proportion of patients who achieved a reduction in kidney inflammation. Data from this trial were also used for the assessment of side effects.
In Trial 2, patients were randomly assigned to receive by mouth one of two doses of LUPKYNIS or placebo for 48 weeks. Neither the patient nor the healthcare providers knew which medication was being given. Data from the trial were used for the assessment of side effects.
How were the trials designed?
There were two trials that provided data for LUPKYNIS approval in SLE patients with biopsy-proven active Class III or IV LN (alone or in combination with Class V LN) or Class V LN.
Trial 1 was a 52 week, double-blind, placebo-controlled trial in patients randomized in a 1:1 ratio to receive either LUPKYNIS 23.7 mg twice daily or placebo on background treatment with mycophenolate mofetil and corticosteroids.
The primary efficacy endpoint was the proportion of patients achieving complete renal response at Week 52. Complete renal response was defined as follows (both must be met):
- UPCR of ≤0.5 mg/mg, and
- eGFR ≥ 60 mL/min/1.73 m2 or no confirmed decrease from baseline in eGFR of > 20% or no treatment- or disease-related eGFR-associated event (defined as blood creatinine increased, creatinine renal clearance decreased, glomerular filtration rate decreased, serum creatinine increased, renal impairment, renal failure, or renal failure acute) at time of assessment.
In order to be considered a responder, the patient must not have received more than 10 mg prednisone for ≥3 consecutive days or for ≥7 days in total during Weeks 44 through 52. Patients who received rescue medication or withdrew from the study were considered non-responders.
Trial 2 was a 48 week, double-blind, placebo-controlled trial in patients randomized in a 1:1:1 ratio to receive either LUPKYNIS 23.7 mg twice daily, voclosporin 39.5 mg twice daily or placebo on background treatment with mycophenolate mofetil and corticosteroids. Data from this trial were used for safety assessment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.