Drug Trials Snapshot: NEXOBRID
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the NEXOBRID Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
NEXOBRID (anacaulase-bcdb)
(nex' oh brid)
MediWound, Ltd.
Original Approval date: December 28, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NEXOBRID is a mixture of enzymes extracted from the stems of pineapple plants that is indicated for removal of charred tissue in adults with deep second degree and/or third degree burns from heat sources, such as flames or scalds from hot liquids.
How is this drug used?
NEXOBRID is only to be administered by a healthcare provider. It is applied to a burn wound of up to 15% of total skin surface and removed after 4 hours. It may be applied a second time if all charred tissue was not removed from the first application.
Who participated in the clinical trials?
The FDA approved NEXOBRID based on evidence from two clinical trials of 357 patients with deep partial thickness (DPT) and/or full thickness (FT) thermal burns. The trials were conducted in 15 countries including the United States, Romania, Italy, Georgia, Czech Republic, Belgium, Germany, France, Slovakia, Poland, United Kingdom, Australia, India, and Israel. Among the 357 enrolled subjects, 350 subjects were evaluated for safety and 331 subjects were evaluated for efficacy (Intent-to-Treat population).
How were the trials designed?
NEXOBRID was evaluated in two clinical trials for eschar removal in patients with DPT and/or FT thermal burns. Study 1 was a randomized three-arm study, comparing NEXOBRID, Standard of Care (SOC), and gel vehicle treatment. Study 2 was a randomized, two-arm study, comparing NEXOBRID to SOC treatment. The healthcare provider applied NEXOBRID or gel vehicle (in Study 1 only) to the treatment area one or two times or used either surgical or non-surgical SOC methods for eschar removal. Any eschar that remained after NEXOBRID or gel vehicle treatment was removed with surgical or non-surgical SOC methods. In Study 1, a separate assessor not involved with treatment was used to determine the amount of eschar remaining after the topical treatment period.
Efficacy was assessed as the incidence of ≥95% eschar removal at the end of the topical treatment period for subjects in the NEXOBRID and gel vehicle groups and as the incidence of surgical methods for eschar removal for the NEXOBRID and SOC groups.
How were the trials designed?
NEXOBRID was evaluated in two clinical trials for eschar removal in patients with DPT and/or FT thermal burns.
Study 1 was a randomized three-arm study, comparing NEXOBRID, SOC, and gel vehicle treatment in adult patients with DPT and/or FT thermal burns of 3% to 30% body surface area. The health care provider applied NEXOBRID or gel vehicle to the treatment area one or two times or used either surgical or non-surgical SOC methods for eschar removal. Any eschar that remained after NEXOBRID or gel vehicle treatment was removed with surgical or non-surgical SOC methods. A separate assessor not involved with treatment was used to determine the amount of eschar remaining after the topical treatment period. The study enrolled 175 patients. Efficacy was assessed as the incidence of ≥95% eschar removal at the end of the topical treatment period for subjects in the NEXOBRID and gel vehicle groups and as the incidence of surgical methods for eschar removal for the NEXOBRID and SOC groups.
Study 2 was a randomized, two-arm study, comparing NEXOBRID to SOC treatment in patients with DPT and/or FT thermal burns of 5% to 24% body surface area. The health care provider applied NEXOBRID to the treatment area one or two times or used either surgical or non-surgical SOC methods for eschar removal. Any eschar that remained after NEXOBRID treatment was removed with surgical or non-surgical SOC methods. In Study 2, both the health care provider and the patient were aware of which treatment was used. The study enrolled 182 subjects. The first patient at each site (26 subjects) was not randomized and was treated with NEXOBRID. The remaining 156 subjects were randomized to NEXOBRID or SOC. Efficacy was assessed as the incidence of use of surgical methods for eschar removal for the NEXOBRID and SOC groups on DPT burns only (97 patients).
DEMOGRAPHICS SNAPSHOT:
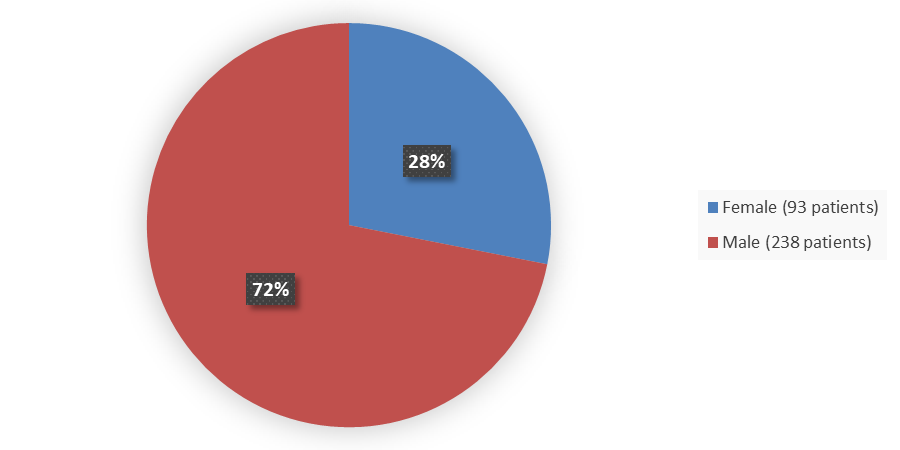
Figure 1 summarizes how many males and females were enrolled in the combined clinical trials used to evaluate the efficacy of NEXOBRID.
Figure 1. Baseline Demographics by Sex (Intent-to-Treat Population)
Source: Adapted from FDA Review
Figure 2 summarizes the percentage of patients by race enrolled in the combined clinical trials used to evaluate the efficacy of NEXOBRID.
Figure 2. Baseline Demographics by Race (Intent-to-Treat Population)
Source: Adapted from FDA Review
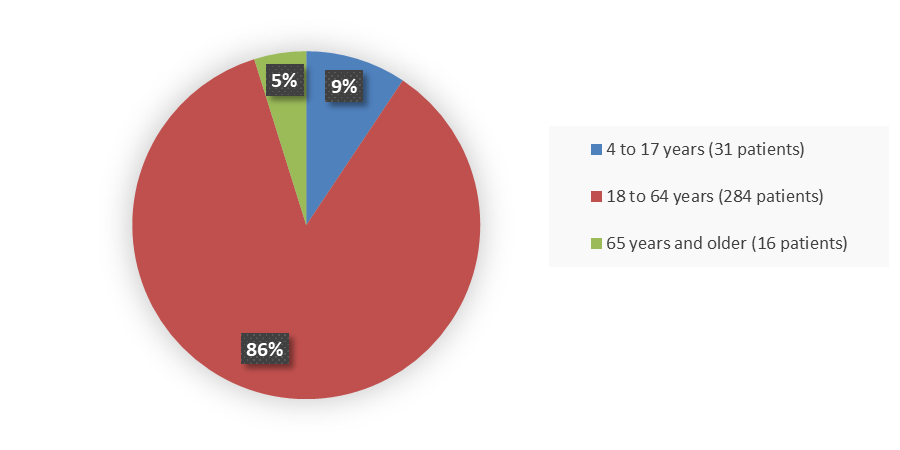
Figure 3 summarizes how many patients by age were enrolled in the combined clinical trials used to evaluate the efficacy of NEXOBRID.
Figure 3. Baseline Demographics by Age (Intent-to-Treat Population)
Source: Adapted from FDA Review
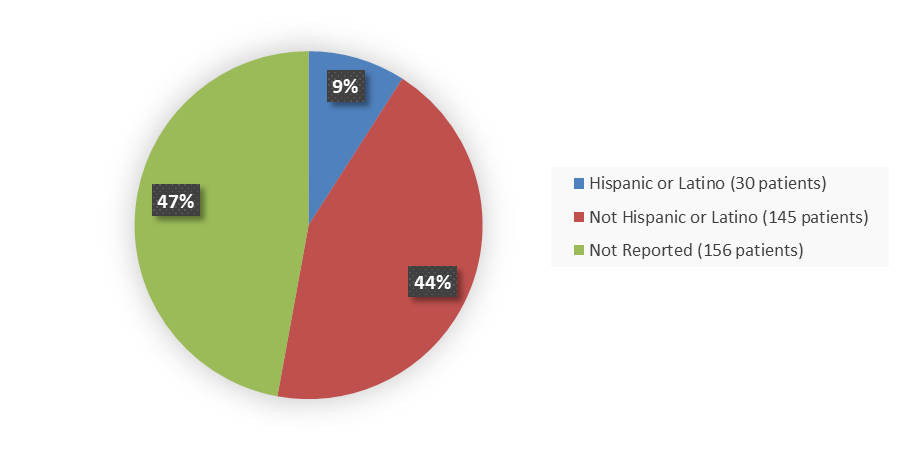
Figure 4 summarizes how many patients by ethnicity were enrolled in the combined clinical trials used to evaluate the efficacy of NEXOBRID.
Figure 4. Baseline Demographics by Ethnicity (Intent-to-Treat Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 5 summarizes the demographics in the intent-to-treat population in Studies 1 and 2.
Table 5. Demographics (Intent-to-Treat)
|
Demographic |
Study 1 N=175 n (%) |
Study 2 N=156 n (%) |
|---|---|---|
|
Age, years |
|
|
|
4 to <18 |
-- |
31 (20) |
|
18 to <65 |
159 (91) |
125 (80) |
|
≥65 |
16 (9) |
-- |
|
Sex |
|
|
|
Female |
52 (30) |
41 (26) |
|
Male |
123 (70) |
115 (74) |
|
Race |
|
|
|
Asian |
2 (1) |
8 (5) |
|
Black or African American |
24 (14) |
9 (6) |
|
White |
141 (81) |
128 (82) |
|
Other |
8 (5) |
11 (7) |
|
Ethnicity |
|
|
|
Not Hispanic or Latino |
145 (83) |
Not Reported |
|
Hispanic or Latino |
30 (17) |
Not Reported |
Source: Adapted from FDA Review
What are the benefits of this drug?
NEXOBRID removes charred tissues (eschar) from serious burn wounds from heat sources, such as flames or scalds from hot liquids.
What are the benefits of this drug (results of trials used to assess efficacy)?
Studies 1 and 2 evaluated eschar removal in subjects with DPT and/or FT thermal burns. Subjects in Study 1 were randomized to NEXOBRID, SOC, or gel vehicle. Subjects in Study 2 were randomized to NEXOBRID or SOC.
Study 1 evaluated the proportions of subjects with ≥95% eschar removal at the end of the topical treatment period (NEXOBRID versus gel vehicle; Table 1). Studies 1 and 2 evaluated the proportion of subjects needing surgical excision for eschar removal (NEXOBRID versus SOC; Table 2). In Study 2, efficacy was assessed on DPT burns only.
Table 1. Proportion of Subjects With ≥95% Eschar Removal at the End of the Topical Treatment Period (Study 1)
|
Subjects With ≥95% Eschar Removal |
NEXOBRID N=75 n (%) |
Gel Vehicle N=25 n (%) |
Treatment Difference % (95% CI) |
|---|---|---|---|
|
Study 1 |
70 (93) |
1 (4) |
89 (74, 96) |
Source: NEXOBRID Prescribing Information
Abbreviations: CI, confidence interval
Table 2. Proportion of Subjects With Excision for Eschar Removal
|
Subjects With Excision for Eschar Removal |
NEXOBRID n/N (%) |
SOC n/N (%) |
Treatment Difference % (95% CI) |
|---|---|---|---|
|
Study 1 (DPT or FT) |
3/75 (4) |
54/75 (72) |
-68 (-78, -56) |
|
Study 2 (DPT) |
11/49 (22) |
37/48 (77) |
-55 (-71, -38) |
Source: NEXOBRID Prescribing Information
Abbreviations: CI, confidence interval; DPT, deep partial thickness; FT, full thickness; SOC, standard of care
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: NEXOBRID worked similarly in males and females.
- Race: The observed effect of NEXOBRID worked similarly in White and Black or African American patients, however the number of patients of races other than White was small; therefore, differences in how NEXOBRID worked among races could not be determined.
- Age: The number of patients 65 years of age and older was small; therefore, differences in how NEXOBRID worked across age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The proportions of subjects who needed surgical excision for eschar removal (NEXOBRID versus SOC) by subgroup among subjects with DPT and FT wounds in the two studies are shown in Table 3.
Table 3. Proportion of Subjects With Excision for Eschar Removal by Subgroup in DPT and FT Wounds
|
Demographic |
Study 1 (DPT/FT) |
Study 2 (DPT/FT) |
||
|---|---|---|---|---|
|
NEXOBRID N=75 n/Ns (%) |
SOC N=75 n/Ns (%) |
NEXOBRID N=75 n/Ns (%) |
SOC N=81 n/Ns (%) |
|
|
Age, years |
|
|
|
|
|
4 to <18 |
-- |
-- |
4/15 (28) |
13/16 (81) |
|
18 to <65 |
2/69 (3) |
51/69 (74) |
20/60 (33) |
52/65 (80) |
|
≥65 |
1/6 (17) |
3/6 (50) |
-- |
-- |
|
Sex |
|
|
|
|
|
Female |
1/26 (4) |
11/16 (69) |
10/21 (48) |
15/20 (75) |
|
Male |
2/49 (4) |
43/59 (73) |
14/54 (26) |
50/61 (82) |
|
Race |
|
|
|
|
|
Asian |
0/1 (0) |
1/1 (100) |
0/5 (0) |
3/3 (100) |
|
Black or African American |
0/8 (0) |
11/13 (85) |
1/4 (25) |
3/5 (60) |
|
White |
3/61 (5) |
41/59 (69) |
23/63 (37) |
52/65 (80) |
|
Other |
0/5 (0) |
1/2 (50) |
0/3 (0) |
7/8 (88) |
|
Ethnicity |
|
|
|
|
|
Not Hispanic or Latino |
2/61 (3) |
51/67 (76) |
Not reported |
Not reported |
|
Hispanic or Latino |
1/14 (7) |
3/8 (38) |
Not reported |
Not reported |
Source: Adapted from FDA review
Abbreviations: DPT, deep partial thickness; FT, full thickness; n, number of subjects with excision; Nz, number of subjects in subgroup for each treatment arm; SOC, standard of care
What are the possible side effects?
The most common side effects in the trials (occurred in more than 10% of study subjects) were itching and fever. Serious allergic reactions, including life-threatening allergic reactions, have been reported with use of NEXOBRID. Pain control is important all through the NEXOBRID process. Use of NEXOBRID should be avoided in patients on blood thinners or patients who are prone to easy bleeding.
What are the possible side effects (results of trials used to assess safety)?
Safety was evaluated in 350 subjects (NEXOBRID, SOC, or gel vehicle) with deep partial thickness and/or full thickness thermal burns in two studies (Studies 1 and 2).
Table 4 presents adverse reactions reported in ≥1% and greater incidence than SOC in NEXOBRID-treated subjects for eschar removal in deep partial thickness and/or full thickness thermal burns among patients treated with NEXOBRID or SOC.
Table 4. Adverse Reactions Reported in ≥1% and Greater Incidence Than Standard of Care in NEXOBRID-Treated Patients for Eschar Removal in Deep Partial Thickness and/or Full Thickness Thermal Burns in Studies 1 and 2a
|
Adverse Reaction |
NEXOBRID N=177 n (%) |
Standard of Careb N=149 n (%) |
|---|---|---|
|
Pruritus |
27 (15) |
19 (13) |
|
Pyrexia |
21 (12) |
13 (9) |
|
Wound complicationc |
15 (9) |
9 (6) |
|
Anemia |
11 (6) |
8 (5) |
|
Vomiting |
9 (5) |
4 (3) |
|
Insomnia |
8 (5) |
6 (4) |
|
Urinary tract infection |
7 (4) |
1 (1) |
|
Tachycardia |
5 (3) |
0 |
|
Rash |
6 (3) |
0 |
|
Infection |
4 (2) |
2 (1) |
|
Sepsis |
4 (2) |
1 (1) |
|
Leukocytosis |
3 (2) |
1(1) |
|
Hypotension |
3 (2) |
1 (1) |
|
Hepatic function abnormal |
2 (1) |
0 |
|
Drug hypersensitivity |
2 (1) |
0 |
|
Bacteremia |
2 (1) |
0 |
|
Scar |
2 (1) |
0 |
|
Subcutaneous hematoma |
2 (1) |
0 |
|
Decubitus ulcer |
2 (1) |
0 |
Source: NEXOBRID Prescribing Information
a During the time period from baseline to 3 months post-wound closure
b Standard of Care treatment included both surgical and non-surgical eschar removal methods
c Wound complication includes skin graft failure, graft loss, graft complication, and wound decomposition
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in males or females.
- Race: The number of patients of races other than White was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The number of subjects 65 years of age and older was small; therefore, differences in the occurrence of side effects across age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Among male subjects, approximately 66% of NEXOBRID-treated patients experienced an adverse event versus 55% of SOC-treated patients. Among female subjects, approximately 60% of NEXOBRID-treated patients experienced an adverse event versus 54% of SOC-treated patients. The number of patients of races other than White was small. The number of patients 65 years of age and older was small. Therefore, differences in the occurrence of side effects among races or age groups could not be determined.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION