Drug Trials Snapshot: OMLONTI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the OMLONTI Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
OMLONTI (omidenepag Isopropyl)
(om lon’ tee)
Santen Inc.
Original Approval date: September 22, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
OMLONTI is a treatment that reduces the pressure inside the eye in patients with elevated (high) eye pressure or open-angle glaucoma. High eye pressure is a factor in the development of permanent optic nerve changes. Medical treatment includes lowering eye pressure below the level that is likely to produce further damage to the optic nerve.
How is this drug used?
OMLONTI is an eyedrop that is taken once daily in the evening in each eye that needs eye pressure control.
Who participated in the clinical trials?
The FDA approved OMLONTI based on evidence from 3 clinical trials of 1,203 patients with open-angle glaucoma or ocular hypertension. The trials were conducted at 112 of sites in 5 countries in the United States, India, Taiwan, Korea, and Singapore.
How were the trials designed?
OMLONTI was evaluated in 3 clinical trials of 1,203 patients with open-angle glaucoma (OAG) or ocular hypertension (OHT). The three studies were all multicenter, double-masked, randomized, parallel-group, active-controlled, non-inferiority studies. The primary objective of these studies was to evaluate the safety and efficacy of OMLONTI compared with timolol 0.5% twice daily (Trial 1 and Trial 2) or with latanoprost 0.005% once daily (Trial 3) in subjects with OAG and OHT.
For Trials 1 and 2, the primary efficacy endpoint was intraocular pressure (IOP) in the study eye measured at three scheduled times of the day (08:00, 10:00, and 16:00) on each of the three follow-up visits, Week 1, Week 6, and Month 3 (i.e., IOP at 9 measurement timepoints). For Trial 3, the primary efficacy endpoint was the mean diurnal IOP (average of IOP at 3 time points: 09:00, 13:00, and 17:00) at Month 3. However, to meet the FDA’s requirement, this study evaluated IOP at three scheduled timepoints (09:00, 13:00, and 17:00) at Week 1, Week 6, and Month 3 (i.e., IOP at 9 measurement timepoints) as an alpha-adjusted key-secondary efficacy endpoint. This endpoint is consistent with endpoints considered for this indication where latanoprost is used as an active comparator.
DEMOGRAPHICS SNAPSHOT:
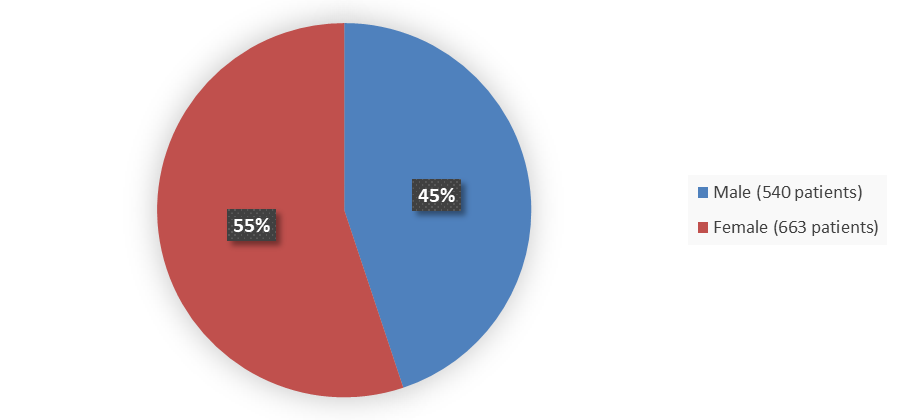
Figure 1. Baseline Demographics by Sex Trials ISS (011709IN, 011710IN, 01171505)
Source: Adapted from FDA Review
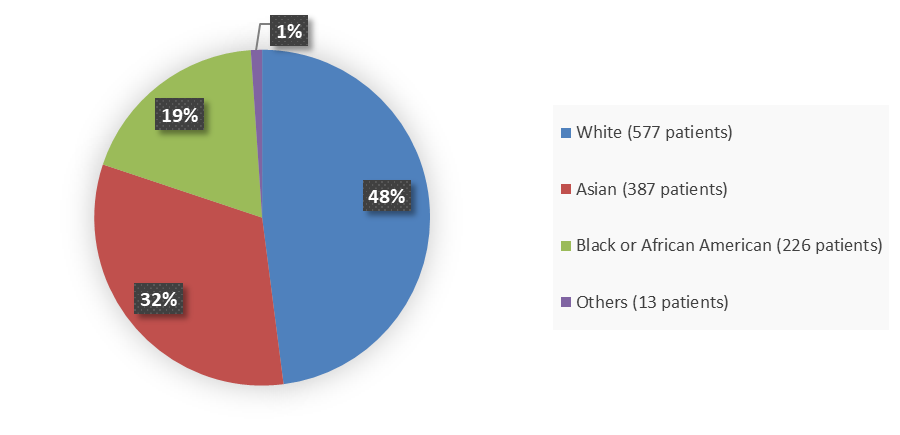
Figure 2. Baseline Demographics for Race, Trials ISS (011709IN, 011710IN, 01171505)
Source: Adapted from FDA Review
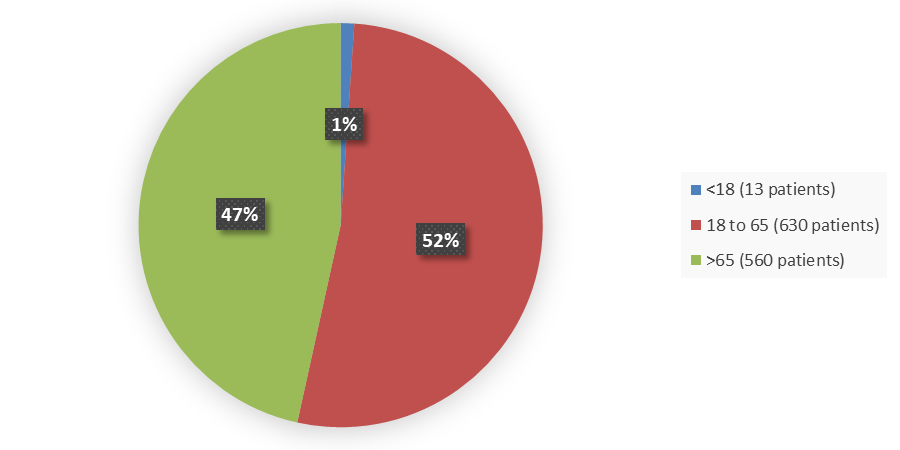
Figure 3. Baseline Demographics for Age, Trials ISS (011709IN, 011710IN, 01171505)
Source: Adapted from FDA Review
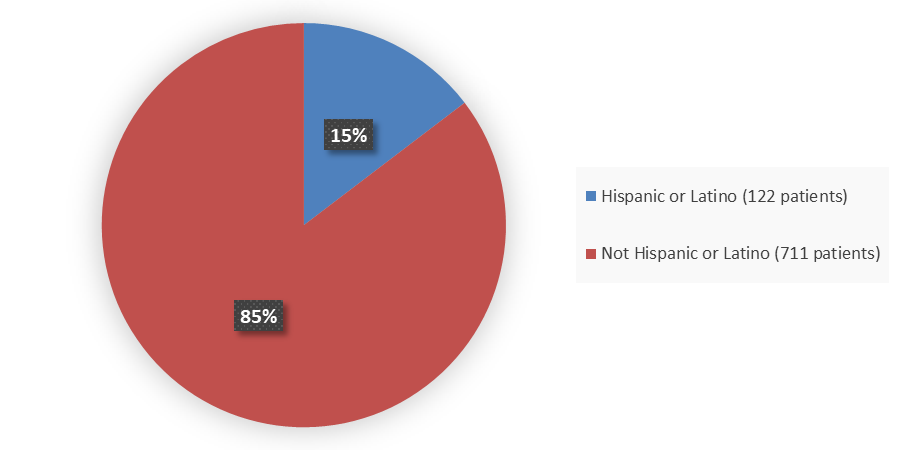
Figure 4. Baseline Demographics by Ethnicity (Full Analysis Set)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Demographics of Efficacy Trials by Race (Full Analysis Set)
|
Trial 1 |
Trial 2 |
Trial 3 |
||||
|
OMLONTI N=212 n (%) |
Timolol N=213 n (%) |
OMLONTI N=204 n (%) |
Timolol N=205 n (%) |
OMLONTI N=184 n (%) |
Latano N=185 n (%) |
|
|
Race |
|
|
|
|
|
|
|
American Indian or Alaska Native |
0 (0.0) |
1 (0.5) |
0 (0.0) |
1 (0.5) |
0 (0.0) |
0 (0.0) |
|
Asian |
0 (0.0) |
2 (0.9) |
8 (3.9) |
8 (3.9) |
184 (100) |
185 (100) |
|
Black or African American |
48 (22.6) |
52 (24.4) |
72 (35.3) |
54 (26.3) |
0 (0.0) |
0 (0.0) |
|
Native Hawaiian or Other Pacific Islander |
2 (0.9) |
0 (0.0) |
0 (0.0) |
1 (0.5) |
0 (0.0) |
0 (0.0) |
|
White |
160 (75.5) |
155 (72.8) |
122 (59.8) |
140 (68.3) |
0 (0.0) |
0 (0.0) |
|
Other |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
Multiple |
2 (0.9) |
3 (1.4) |
2 (1.0) |
1 (0.5) |
0 (0.0) |
0 (0.0) |
Source: Table 13 (Study 011709IN) and Table 12 (Study 011710IN) and Table 6 (01171505) of the study reports.
Abbreviations: Latano= latanoprost
What are the benefits of this drug?
In the three clinical trials that evaluated OMLONTI after three months of treatment, OMLONTI reduced eye pressure by an amount that was no different from the drugs used as controls. Both OMLONTI and the controls reduced eye pressure by a medically important amount.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2, Table 3, and Table 4 summarize the efficacy results for the individual Trials 1, 2, and 3 for the elevated eye pressure indication. The primary endpoints of the trials were average eye pressure measured at multiple time points over three months.
Table 2. Primary Efficacy Endpoint Summary - LS Means and 95% CI, Trial 1
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
Baseline (8:00) |
25.3 (0.19) |
25.5 (0.19) |
-0.18 (-0.7, 0.35) |
|
Baseline (10:00) |
24.7 (0.16) |
24.6 (0.16) |
0.05 (-0.41, 0.51) |
|
Baseline (16:00) |
24.2 (0.15) |
24.4 (0.15) |
-0.19 (-0.61, 0.23) |
|
Week 1 (8:00) |
19 (0.22) |
19.1 (0.21) |
-0.1 (-0.7, 0.5) |
|
Week 1 (10:00) |
18 (0.21) |
18.2 (0.21) |
-0.2 (-0.8, 0.4) |
|
Week 1 (16:00) |
17.5 (0.21) |
17.9 (0.21) |
-0.4 (-1, 0.1) |
|
Week 6 (8:00) |
19.8 (0.2) |
18.4 (0.2) |
1.3 (0.8, 1.9) |
|
Week 6 (10:00) |
18.9 (0.2) |
18 (0.2) |
0.8 (0.3, 1.4) |
|
Week 6 (16:00) |
18.5 (0.21) |
17.7 (0.21) |
0.8 (0.2, 1.4) |
|
Month 3 (8:00) |
19.7 (0.24) |
18.5 (0.24) |
1.2 (0.5, 1.9) |
|
Month 3 (10:00) |
18.8 (0.21) |
17.7 (0.21) |
1.1 (0.5, 1.7) |
|
Month 3 (16:00) |
18.6 (0.22) |
17.8 (0.21) |
0.8 (0.2, 1.4) |
Source: NEXOBRID Prescribing Information
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; LS, least squares; SE, standard error
Table 3. Primary Efficacy Endpoint Summary - LS Means and 95% CI, Trial 2
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
Baseline (8:00) |
25.9 (0.2) |
25.5 (0.2) |
0.44 (-0.11, 0.99) |
|
Baseline (10:00) |
25.1 (0.18) |
24.8 (0.18) |
0.24 (-0.26, 0.74) |
|
Baseline (16:00) |
24.7 (0.17) |
24.2 (0.17) |
0.46 (-0.01, 0.92) |
|
Week 1 (8:00) |
19.4 (0.23) |
19.7 (0.23) |
-0.3 (-0.9, 0.3) |
|
Week 1 (10:00) |
18.5 (0.22) |
18.9 (0.22) |
-0.4 (-1, 0.3) |
|
Week 1 (16:00) |
17.9 (0.23) |
18.6 (0.22) |
-0.6 (-1.3, 0) |
|
Week 6 (8:00) |
20.4 (0.22) |
19.5 (0.21) |
0.9 (0.3, 1.5) |
|
Week 6 (10:00) |
19.5 (0.2) |
18.8 (0.2) |
0.7 (0.1, 1.2) |
|
Week 6 (16:00) |
19.2 (0.21) |
18.8 (0.21) |
0.3 (-0.2, 0.9) |
|
Month 3 (8:00) |
20 (0.22) |
19.6 (0.22) |
0.4 (-0.2, 1) |
|
Month 3 (10:00) |
19.4 (0.23) |
18.9 (0.22) |
0.5 (-0.1, 1.1) |
|
Month 3 (16:00) |
19.1 (0.23) |
19 (0.22) |
0.1 (-0.5, 0.7) |
Source: Adapted from FDA Review
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; LS, least squares; SE, standard error
Table 4. Primary Efficacy Endpoint Summary - LS Means and 95% CI, Trial 3
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Latanoprost |
||
|
Baseline (9:00) |
24.9 (0.18) |
24.7 (0.18) |
0.15 (-0.36, 0.65) |
|
Baseline (13:00) |
24.5 (0.17) |
24.5 (0.17) |
0.05 (-0.43, 0.52) |
|
Baseline (17:00) |
24.3 (0.17) |
24.3 (0.17) |
0.03 (-0.45, 0.51) |
|
Week 1 (9:00) |
19 (0.28) |
18.8 (0.29) |
0.2 (-0.5, 0.9) |
|
Week 1 (13:00) |
18.4 (0.28) |
18.4 (0.29) |
0 (-0.7, 0.7) |
|
Week 1 (17:00) |
18 (0.28) |
18.3 (0.28) |
-0.2 (-0.9, 0.5) |
|
Week 6 (9:00) |
17.8 (0.26) |
17.4 (0.26) |
0.4 (-0.3, 1) |
|
Week 6 (13:00) |
17.6 (0.27) |
17.2 (0.27) |
0.4 (-0.3, 1) |
|
Week 6 (17:00) |
17.5 (0.26) |
17.1 (0.27) |
0.4 (-0.3, 1) |
|
Month 3 (9:00) |
17.9 (0.27) |
17 (0.27) |
0.9 (0.2, 1.5) |
|
Month 3 (13:00) |
17.2 (0.25) |
16.7 (0.26) |
0.6 (0, 1.2) |
|
Month 3 (17:00) |
17.2 (0.26) |
16.7 (0.27) |
0.5 (-0.2, 1.1) |
Source: Adapted from FDA Review
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Latanoprost
Abbreviations: CI, confidence interval; LS, least squares; SE, standard error
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: OMLONTI worked similarly in males and females.
- *Race: OMLONTI worked similarly in White and Black or African American patients.
- Age: OMLONTI worked similarly in patients younger and older than 65 years of age.
*In Trials 1 and 2, the number of patients of races other than White and Black was small; therefore, differences in how OMLONTI worked among races could not be determined. Trial 3 was exclusively conducted in Asia therefore analysis by race is not conducted for this study.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 5. Subgroup Analysis by Sex, Trial 1
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
Female |
|
|
|
|
Baseline (8:00) |
25.5 (0.25) |
25.3 (0.24) |
0.22 (-0.45, 0.89) |
|
Baseline (10:00) |
24.7 (0.21) |
24.5 (0.2) |
0.25 (-0.33, 0.83) |
|
Baseline (16:00) |
24.2 (0.19) |
24.1 (0.18) |
0.08 (-0.43, 0.59) |
|
Week 1 (8:00) |
19.3 (0.28) |
19.5 (0.26) |
-0.2 (-0.9, 0.6) |
|
Week 1 (10:00) |
18.2 (0.26) |
18.4 (0.25) |
-0.2 (-0.9, 0.5) |
|
Week 1 (16:00) |
17.6 (0.27) |
18 (0.26) |
-0.3 (-1, 0.4) |
|
Week 6 (8:00) |
19.9 (0.25) |
18.7 (0.24) |
1.2 (0.5, 1.8) |
|
Week 6 (10:00) |
18.8 (0.25) |
18.3 (0.23) |
0.6 (-0.1, 1.2) |
|
Week 6 (16:00) |
18.3 (0.27) |
17.9 (0.25) |
0.4 (-0.3, 1.1) |
|
Month 3 (8:00) |
19.8 (0.31) |
18.7 (0.29) |
1.1 (0.2, 1.9) |
|
Month 3 (10:00) |
18.6 (0.26) |
17.7 (0.24) |
1 (0.3, 1.7) |
|
Month 3 (16:00) |
18.6 (0.28) |
18 (0.26) |
0.6 (-0.1, 1.4) |
|
Male |
|
|
|
|
Baseline (8:00) |
25 (0.29) |
25.8 (0.31) |
-0.79 (-1.64, 0.06) |
|
Baseline (10:00) |
24.6 (0.26) |
24.9 (0.28) |
-0.27 (-1.03, 0.48) |
|
Baseline (16:00) |
24.1 (0.25) |
24.8 (0.27) |
-0.67 (-1.39, 0.06) |
|
Week 1 (8:00) |
18.6 (0.34) |
18.4 (0.36) |
0.1 (-0.9, 1.1) |
|
Week 1 (10:00) |
17.6 (0.35) |
17.7 (0.38) |
-0.2 (-1.2, 0.9) |
|
Week 1 (16:00) |
17.3 (0.32) |
17.8 (0.34) |
-0.5 (-1.4, 0.4) |
|
Week 6 (8:00) |
19.6 (0.33) |
17.9 (0.36) |
1.7 (0.8, 2.7) |
|
Week 6 (10:00) |
18.9 (0.35) |
17.6 (0.37) |
1.3 (0.3, 2.3) |
|
Week 6 (16:00) |
18.8 (0.34) |
17.3 (0.36) |
1.4 (0.4, 2.4) |
|
Month 3 (8:00) |
19.7 (0.38) |
18.1 (0.41) |
1.5 (0.4, 2.6) |
|
Month 3 (10:00) |
18.9 (0.35) |
17.6 (0.37) |
1.3 (0.3, 2.3) |
|
Month 3 (16:00) |
18.7 (0.35) |
17.5 (0.37) |
1.2 (0.2, 2.3) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; SE, standard error
Table 6. Subgroup Analysis by Age Group, Trial 1
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
Age <65 years |
|
|
|
|
Baseline (8:00) |
25.4 (0.29) |
25.6 (0.28) |
-0.22 (-1.02, 0.58) |
|
Baseline (10:00) |
24.8 (0.24) |
24.4 (0.23) |
0.44 (-0.21, 1.09) |
|
Baseline (16:00) |
24 (0.22) |
24.1 (0.21) |
-0.15 (-0.74, 0.44) |
|
Week 1 (8:00) |
19.3 (0.35) |
19.4 (0.33) |
-0.1 (-1.1, 0.8) |
|
Week 1 (10:00) |
18.1 (0.36) |
18.4 (0.34) |
-0.2 (-1.2, 0.7) |
|
Week 1 (16:00) |
17.4 (0.31) |
17.8 (0.3) |
-0.4 (-1.3, 0.5) |
|
Week 6 (8:00) |
19.6 (0.32) |
18.4 (0.3) |
1.2 (0.3, 2.1) |
|
Week 6 (10:00) |
18.6 (0.33) |
18 (0.31) |
0.6 (-0.3, 1.5) |
|
Week 6 (16:00) |
18 (0.31) |
17.7 (0.29) |
0.3 (-0.6, 1.1) |
|
Month 3 (8:00) |
19.6 (0.37) |
18.7 (0.35) |
0.9 (-0.1, 1.9) |
|
Month 3 (10:00) |
18.1 (0.3) |
17.4 (0.29) |
0.7 (-0.1, 1.5) |
|
Month 3 (16:00) |
18 (0.29) |
17.5 (0.27) |
0.4 (-0.4, 1.2) |
|
Age ≥65 years |
|
|
|
|
Baseline (8:00) |
25.2 (0.25) |
25.4 (0.26) |
-0.13 (-0.83, 0.58) |
|
Baseline (10:00) |
24.6 (0.23) |
24.9 (0.23) |
-0.27 (-0.91, 0.37) |
|
Baseline (16:00) |
24.3 (0.21) |
24.6 (0.22) |
-0.26 (-0.85, 0.33) |
|
Week 1 (8:00) |
18.7 (0.27) |
18.8 (0.28) |
-0.1 (-0.9, 0.6) |
|
Week 1 (10:00) |
17.8 (0.26) |
18.1 (0.27) |
-0.2 (-1, 0.5) |
|
Week 1 (16:00) |
17.6 (0.27) |
18 (0.28) |
-0.5 (-1.2, 0.3) |
|
Week 6 (8:00) |
19.9 (0.26) |
18.5 (0.27) |
1.4 (0.7, 2.2) |
|
Week 6 (10:00) |
19.1 (0.26) |
18.1 (0.27) |
1 (0.2, 1.7) |
|
Week 6 (16:00) |
18.9 (0.29) |
17.7 (0.29) |
1.2 (0.4, 2) |
|
Month 3 (8:00) |
19.8 (0.31) |
18.4 (0.32) |
1.4 (0.5, 2.3) |
|
Month 3 (10:00) |
19.2 (0.28) |
17.9 (0.29) |
1.3 (0.6, 2.1) |
|
Month 3 (16:00) |
19.1 (0.31) |
18 (0.32) |
1.1 (0.2, 2) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; SE, standard error
Table 7. Subgroup Analysis by Race, Trial 1
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
White |
|
|
|
|
Baseline (8:00) |
25.2 (0.22) |
25.3 (0.22) |
-0.11 (-0.72, 0.5) |
|
Baseline (10:00) |
24.7 (0.19) |
24.6 (0.19) |
0.08 (-0.44, 0.61) |
|
Baseline (16:00) |
24.2 (0.17) |
24.4 (0.18) |
-0.21 (-0.69, 0.28) |
|
Week 1 (8:00) |
18.9 (0.25) |
19 (0.25) |
-0.1 (-0.8, 0.6) |
|
Week 1 (10:00) |
18 (0.24) |
18.1 (0.24) |
-0.2 (-0.8, 0.5) |
|
Week 1 (16:00) |
17.5 (0.24) |
17.8 (0.25) |
-0.3 (-1, 0.4) |
|
Week 6 (8:00) |
19.9 (0.24) |
18.5 (0.24) |
1.4 (0.7, 2.1) |
|
Week 6 (10:00) |
19 (0.23) |
18 (0.23) |
1 (0.4, 1.7) |
|
Week 6 (16:00) |
18.6 (0.25) |
17.6 (0.25) |
1 (0.3, 1.7) |
|
Month 3 (8:00) |
19.9 (0.29) |
18.6 (0.29) |
1.3 (0.5, 2.1) |
|
Month 3 (10:00) |
19 (0.25) |
17.7 (0.25) |
1.3 (0.6, 2) |
|
Month 3 (16:00) |
18.8 (0.26) |
17.9 (0.26) |
0.9 (0.2, 1.7) |
|
Black or African American |
|
|
|
|
Baseline (8:00) |
25.5 (0.4) |
25.9 (0.39) |
-0.4 (-1.51, 0.71) |
|
Baseline (10:00) |
24.5 (0.35) |
24.8 (0.34) |
-0.31 (-1.27, 0.66) |
|
Baseline (16:00) |
24 (0.31) |
24.3 (0.3) |
-0.31 (-1.18, 0.56) |
|
Week 1 (8:00) |
19.1 (0.45) |
19.4 (0.43) |
-0.3 (-1.5, 1) |
|
Week 1 (10:00) |
18.1 (0.47) |
18.2 (0.45) |
-0.1 (-1.4, 1.2) |
|
Week 1 (16:00) |
17.5 (0.42) |
18.2 (0.4) |
-0.7 (-1.9, 0.4) |
|
Week 6 (8:00) |
19.6 (0.41) |
18.3 (0.39) |
1.3 (0.1, 2.4) |
|
Week 6 (10:00) |
18.5 (0.4) |
17.8 (0.38) |
0.8 (-0.3, 1.8) |
|
Week 6 (16:00) |
18.1 (0.44) |
17.8 (0.42) |
0.4 (-0.8, 1.6) |
|
Month 3 (8:00) |
19.3 (0.44) |
18.3 (0.42) |
1 (-0.2, 2.2) |
|
Month 3 (10:00) |
18.1 (0.36) |
17.3 (0.34) |
0.8 (-0.2, 1.8) |
|
Month 3 (16:00) |
18.3 (0.4) |
17.8 (0.38) |
0.5 (-0.6, 1.6) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; SE, standard error
Table 8. Subgroup Analysis by Sex, Trial 2
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
Female |
|
|
|
|
Baseline (8:00) |
26.1 (0.25) |
25.5 (0.27) |
0.63 (-0.1, 1.36) |
|
Baseline (10:00) |
25.2 (0.24) |
24.7 (0.25) |
0.44 (-0.25, 1.14) |
|
Baseline (16:00) |
24.7 (0.2) |
24.1 (0.21) |
0.58 (0.01, 1.16) |
|
Week 1 (8:00) |
19.9 (0.27) |
20.2 (0.29) |
-0.3 (-1.1, 0.5) |
|
Week 1 (10:00) |
18.9 (0.26) |
19.3 (0.28) |
-0.4 (-1.2, 0.3) |
|
Week 1 (16:00) |
18.3 (0.27) |
19.1 (0.29) |
-0.9 (-1.7, -0.1) |
|
Week 6 (8:00) |
20.6 (0.27) |
19.6 (0.28) |
1 (0.3, 1.8) |
|
Week 6 (10:00) |
19.6 (0.24) |
18.8 (0.25) |
0.7 (0, 1.4) |
|
Week 6 (16:00) |
19.3 (0.25) |
19 (0.26) |
0.3 (-0.5, 1) |
|
Month 3 (8:00) |
20.3 (0.28) |
19.7 (0.29) |
0.6 (-0.2, 1.4) |
|
Month 3 (10:00) |
19.8 (0.26) |
18.9 (0.28) |
0.8 (0.1, 1.6) |
|
Month 3 (16:00) |
19.4 (0.25) |
19.1 (0.27) |
0.3 (-0.4, 1) |
|
Male |
|
|
|
|
Baseline (8:00) |
25.6 (0.31) |
25.5 (0.29) |
0.16 (-0.68, 1) |
|
Baseline (10:00) |
24.9 (0.27) |
24.9 (0.25) |
-0.02 (-0.75, 0.7) |
|
Baseline (16:00) |
24.7 (0.29) |
24.4 (0.27) |
0.32 (-0.45, 1.1) |
|
Week 1 (8:00) |
18.8 (0.39) |
19.2 (0.36) |
-0.4 (-1.5, 0.6) |
|
Week 1 (10:00) |
18 (0.38) |
18.4 (0.35) |
-0.4 (-1.4, 0.6) |
|
Week 1 (16:00) |
17.5 (0.37) |
18 (0.35) |
-0.5 (-1.5, 0.5) |
|
Week 6 (8:00) |
20.2 (0.36) |
19.5 (0.33) |
0.7 (-0.3, 1.7) |
|
Week 6 (10:00) |
19.5 (0.35) |
18.8 (0.32) |
0.6 (-0.3, 1.6) |
|
Week 6 (16:00) |
19 (0.36) |
18.6 (0.33) |
0.4 (-0.6, 1.4) |
|
Month 3 (8:00) |
19.5 (0.37) |
19.4 (0.34) |
0.1 (-0.9, 1.1) |
|
Month 3 (10:00) |
18.9 (0.39) |
18.9 (0.36) |
0.1 (-1, 1.1) |
|
Month 3 (16:00) |
18.8 (0.41) |
19 (0.37) |
-0.2 (-1.3, 0.9) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; SE, standard error
Table 9. Subgroup Analysis by Age Group, Trial 2
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
Age <65 years |
|
|
|
|
Baseline (8:00) |
25.8 (0.3) |
25.5 (0.31) |
0.34 (-0.52, 1.19) |
|
Baseline (10:00) |
24.9 (0.26) |
24.6 (0.26) |
0.25 (-0.48, 0.99) |
|
Baseline (16:00) |
24.6 (0.25) |
24.2 (0.25) |
0.4 (-0.3, 1.09) |
|
Week 1 (8:00) |
19.8 (0.33) |
20.1 (0.34) |
-0.3 (-1.2, 0.6) |
|
Week 1 (10:00) |
18.6 (0.32) |
19 (0.32) |
-0.4 (-1.3, 0.5) |
|
Week 1 (16:00) |
18.1 (0.33) |
18.4 (0.34) |
-0.3 (-1.2, 0.7) |
|
Week 6 (8:00) |
20.6 (0.32) |
19.7 (0.32) |
0.9 (0, 1.8) |
|
Week 6 (10:00) |
19.7 (0.26) |
18.5 (0.26) |
1.1 (0.4, 1.9) |
|
Week 6 (16:00) |
19.2 (0.29) |
18.2 (0.29) |
1 (0.2, 1.8) |
|
Month 3 (8:00) |
19.8 (0.33) |
19.8 (0.33) |
0 (-0.9, 0.9) |
|
Month 3 (10:00) |
19 (0.3) |
18.8 (0.31) |
0.2 (-0.7, 1) |
|
Month 3 (16:00) |
19.2 (0.3) |
19 (0.3) |
0.2 (-0.6, 1.1) |
|
Age ≥65 years |
|
|
|
|
Baseline (8:00) |
26 (0.26) |
25.5 (0.26) |
0.52 (-0.2, 1.24) |
|
Baseline (10:00) |
25.2 (0.25) |
25 (0.24) |
0.24 (-0.44, 0.92) |
|
Baseline (16:00) |
24.8 (0.23) |
24.3 (0.22) |
0.5 (-0.13, 1.13) |
|
Week 1 (8:00) |
19.1 (0.31) |
19.4 (0.31) |
-0.3 (-1.2, 0.6) |
|
Week 1 (10:00) |
18.5 (0.31) |
18.8 (0.3) |
-0.3 (-1.2, 0.5) |
|
Week 1 (16:00) |
17.8 (0.31) |
18.7 (0.3) |
-0.9 (-1.8, -0.1) |
|
Week 6 (8:00) |
20.3 (0.29) |
19.4 (0.29) |
0.9 (0.1, 1.7) |
|
Week 6 (10:00) |
19.4 (0.29) |
19.1 (0.29) |
0.3 (-0.5, 1.1) |
|
Week 6 (16:00) |
19.1 (0.29) |
19.3 (0.29) |
-0.2 (-1, 0.6) |
|
Month 3 (8:00) |
20.1 (0.3) |
19.4 (0.29) |
0.7 (-0.2, 1.5) |
|
Month 3 (10:00) |
19.7 (0.32) |
18.9 (0.31) |
0.8 (-0.1, 1.6) |
|
Month 3 (16:00) |
19.1 (0.33) |
19.1 (0.31) |
0 (-0.9, 0.9) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; SE, standard error
Table 10. Subgroup Analysis by Race, Trial 2
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Timolol |
||
|
White |
|
|
|
|
Baseline (8:00) |
25.9 (0.26) |
25.5 (0.24) |
0.44 (-0.25, 1.13) |
|
Baseline (10:00) |
25 (0.23) |
24.9 (0.22) |
0.03 (-0.6, 0.65) |
|
Baseline (16:00) |
24.9 (0.23) |
24.3 (0.21) |
0.61 (-0.01, 1.22) |
|
Week 1 (8:00) |
18.8 (0.31) |
19.2 (0.29) |
-0.4 (-1.2, 0.4) |
|
Week 1 (10:00) |
18.2 (0.3) |
18.5 (0.28) |
-0.3 (-1.1, 0.5) |
|
Week 1 (16:00) |
17.7 (0.3) |
18.2 (0.28) |
-0.5 (-1.3, 0.3) |
|
Week 6 (8:00) |
20 (0.29) |
19.2 (0.27) |
0.8 (0.1, 1.6) |
|
Week 6 (10:00) |
19.2 (0.27) |
18.8 (0.25) |
0.4 (-0.4, 1.1) |
|
Week 6 (16:00) |
18.8 (0.28) |
18.7 (0.26) |
0.1 (-0.7, 0.8) |
|
Month 3 (8:00) |
20 (0.31) |
19.4 (0.28) |
0.6 (-0.3, 1.4) |
|
Month 3 (10:00) |
19.4 (0.3) |
19 (0.27) |
0.4 (-0.4, 1.2) |
|
Month 3 (16:00) |
19 (0.29) |
18.9 (0.27) |
0.1 (-0.7, 0.9) |
|
Black or African American |
|
|
|
|
Baseline (8:00) |
26.1 (0.34) |
25.7 (0.4) |
0.4 (-0.63, 1.43) |
|
Baseline (10:00) |
25.4 (0.32) |
24.8 (0.36) |
0.59 (-0.36, 1.54) |
|
Baseline (16:00) |
24.6 (0.26) |
24.1 (0.3) |
0.43 (-0.35, 1.22) |
|
Week 1 (8:00) |
20.5 (0.33) |
21.2 (0.39) |
-0.7 (-1.7, 0.3) |
|
Week 1 (10:00) |
19.1 (0.34) |
20.1 (0.39) |
-1 (-2, 0) |
|
Week 1 (16:00) |
18.4 (0.35) |
19.8 (0.41) |
-1.5 (-2.5, -0.4) |
|
Week 6 (8:00) |
21.3 (0.32) |
20.8 (0.37) |
0.5 (-0.5, 1.5) |
|
Week 6 (10:00) |
20.2 (0.31) |
19.4 (0.36) |
0.8 (-0.1, 1.8) |
|
Week 6 (16:00) |
19.8 (0.33) |
19.7 (0.38) |
0.1 (-0.8, 1.1) |
|
Month 3 (8:00) |
20.1 (0.33) |
20.5 (0.38) |
-0.4 (-1.4, 0.6) |
|
Month 3 (10:00) |
19.6 (0.37) |
19.3 (0.42) |
0.3 (-0.8, 1.4) |
|
Month 3 (16:00) |
19.3 (0.39) |
19.9 (0.44) |
-0.6 (-1.8, 0.5) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Timolol
Abbreviations: CI, confidence interval; SE, standard error
Table 11. Subgroup Analysis by Sex, Trial 3
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Latanoprost |
||
|
Female |
|
|
|
|
Baseline (9:00) |
24.7 (0.26) |
24.5 (0.23) |
0.13 (-0.55, 0.82) |
|
Baseline (13:00) |
24.3 (0.25) |
24.3 (0.22) |
0.02 (-0.63, 0.67) |
|
Baseline (17:00) |
24.1 (0.24) |
24 (0.22) |
0.03 (-0.62, 0.67) |
|
Week 1 (9:00) |
19 (0.4) |
18.7 (0.4) |
0.3 (-0.6, 1.3) |
|
Week 1 (13:00) |
18.49 (0.43) |
18.5 (0.43) |
0 (-1, 1) |
|
Week 1 (17:00) |
18 (0.4) |
18.3 (0.4) |
-0.4 (-1.3, 0.6) |
|
Week 6 (9:00) |
17.9 (0.4) |
17.3 (0.4) |
0.7 (-0.3, 1.6) |
|
Week 6 (13:00) |
17.95 (0.41) |
17.2 (0.41) |
0.7 (-0.2, 1.7) |
|
Week 6 (17:00) |
17.7 (0.41) |
16.9 (0.41) |
0.8 (-0.2, 1.8) |
|
Month 3 (9:00) |
18.3 (0.39) |
17 (0.39) |
1.3 (0.4, 2.2) |
|
Month 3 (13:00) |
17.57 (0.38) |
16.6 (0.39) |
1 (0.1, 1.9) |
|
Month 3 (17:00) |
17.5 (0.38) |
16.5 (0.39) |
1 (0.1, 1.9) |
|
Male |
|
|
|
|
Baseline (9:00) |
25 (0.25) |
25 (0.28) |
0.08 (-0.66, 0.83) |
|
Baseline (13:00) |
24.6 (0.24) |
24.6 (0.26) |
0.01 (-0.69, 0.72) |
|
Baseline (17:00) |
24.5 (0.24) |
24.6 (0.26) |
-0.07 (-0.78, 0.63) |
|
Week 1 (9:00) |
18.9 (0.4) |
18.8 (0.43) |
0.1 (-1, 1.2) |
|
Week 1 (13:00) |
18.3 (0.38) |
18.3 (0.41) |
0 (-1, 1) |
|
Week 1 (17:00) |
18 (0.39) |
18.1 (0.41) |
-0.1 (-1.1, 0.9) |
|
Week 6 (9:00) |
17.6 (0.33) |
17.4 (0.35) |
0.1 (-0.7, 1) |
|
Week 6 (13:00) |
17.24 (0.35) |
17.2 (0.36) |
0.1 (-0.8, 1) |
|
Week 6 (17:00) |
17.2 (0.34) |
17.2 (0.36) |
0.1 (-0.8, 0.9) |
|
Month 3 (9:00) |
17.5 (0.38) |
17 (0.4) |
0.5 (-0.4, 1.5) |
|
Month 3 (13:00) |
16.95 (0.34) |
16.7 (0.36) |
0.2 (-0.6, 1.1) |
|
Month 3 (17:00) |
17 (0.36) |
16.9 (0.38) |
0.1 (-0.8, 1) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Lataonprost
Abbreviations: CI, confidence interval; SE, standard error
Table 12. Subgroup Analysis by Age Group, Trial 3
|
Visit (Time)* |
Mean (SE) |
Difference (95% CI) |
|
|
OMLONTI |
Latanoprost |
||
|
Age <65 years |
|
|
|
|
Baseline (9:00) |
24.8 (0.21) |
24.6 (0.2) |
0.24 (-0.33, 0.81) |
|
Baseline (13:00) |
24.5 (0.2) |
24.4 (0.2) |
0.07 (-0.48, 0.63) |
|
Baseline (17:00) |
24.2 (0.19) |
24.2 (0.19) |
-0.03 (-0.56, 0.5) |
|
Week 1 (9:00) |
19.1 (0.39) |
19 (0.4) |
0.1 (-0.8, 0.9) |
|
Week 1 (13:00) |
18.45 (0.38) |
18.6 (0.39) |
-0.2 (-1, 0.7) |
|
Week 1 (17:00) |
18.2 (0.38) |
18.6 (0.39) |
-0.3 (-1.1, 0.5) |
|
Week 6 (9:00) |
18 (0.35) |
17.8 (0.37) |
0.2 (-0.6, 0.9) |
|
Week 6 (13:00) |
17.7 (0.36) |
17.5 (0.37) |
0.2 (-0.6, 0.9) |
|
Week 6 (17:00) |
17.7 (0.36) |
17.5 (0.37) |
0.2 (-0.6, 1) |
|
Month 3 (9:00) |
18.2 (0.36) |
17.3 (0.38) |
0.9 (0.2, 1.7) |
|
Month 3 (13:00) |
17.59 (0.34) |
16.8 (0.35) |
0.7 (0.1, 1.4) |
|
Month 3 (17:00) |
17.6 (0.35) |
16.9 (0.37) |
0.7 (0, 1.4) |
|
Age ≥65 years |
|
|
|
|
Baseline (9:00) |
25 (0.38) |
25.2 (0.4) |
-0.19 (-1.29, 0.9) |
|
Baseline (13:00) |
24.5 (0.34) |
24.6 (0.36) |
-0.05 (-1.03, 0.93) |
|
Baseline (17:00) |
24.7 (0.38) |
24.6 (0.4) |
0.15 (-0.94, 1.24) |
|
Week 1 (9:00) |
19.3 (0.47) |
18.7 (0.52) |
0.5 (-0.8, 1.9) |
|
Week 1 (13:00) |
18.6 (0.48) |
18 (0.54) |
0.6 (-0.8, 2) |
|
Week 1 (17:00) |
18 (0.46) |
17.9 (0.52) |
0.1 (-1.2, 1.4) |
|
Week 6 (9:00) |
17.7 (0.47) |
16.8 (0.52) |
1 (-0.4, 2.3) |
|
Week 6 (13:00) |
17.54 (0.47) |
16.4 (0.51) |
1.1 (-0.2, 2.4) |
|
Week 6 (17:00) |
17.5 (0.5) |
16.5 (0.54) |
1 (-0.4, 2.4) |
|
Month 3 (9:00) |
17.5 (0.52) |
16.9 (0.57) |
0.6 (-0.9, 2) |
|
Month 3 (13:00) |
16.61 (0.52) |
16.5 (0.56) |
0.2 (-1.3, 1.6) |
|
Month 3 (17:00) |
16.6 (0.51) |
16.7 (0.56) |
-0.1 (-1.5, 1.3) |
Source: Adapted from FDA Review.
* Time of measurement is recorded in 24-hour format
Difference = OMLONTI - Latanoprost
Abbreviations: CI, confidence interval; SE, standard error
What are the possible side effects?
The most common adverse reactions seen in at least 1% of treated patients were eye redness, light sensitivity, blurred vision, dry eye sensation, eye irritation, and headache.
OMLONTI belongs to a class of eyedrops that may increase eye pigmentation and eyelash pigmentation. This class of drug may also cause inflammation and swelling inside the eye.
What are the possible side effects (results of trials used to assess safety)?
Table 13. Patients With Common Adverse Events Occurring at ≥1% Frequency, Safety Population, Trials ISS (011709IN, 011710IN, 01171505)
|
Preferred Term |
Trial 1 (011709IN) |
Trial 2 (011710IN) |
Trial 3 (01171505) |
|||
|
OMLONTI |
Timolol |
OMLONTI |
Timolol |
OMLONTI |
Latanoprost |
|
|
Any AE |
88 (41.7) |
77 (35.8) |
84 (41.2) |
67 (32.7) |
74 (40.0) |
55 (29.7) |
|
Conjunctival hyperaemia |
13 (6.2) |
9 (4.2) |
16 (7.8) |
7 (3.4) |
22 (11.9) |
10 (5.4) |
|
Vision blurred |
11 (5.2) |
3 (1.4) |
6 (2.9) |
2 (1.0) |
4 (2.2) |
2 (1.1) |
|
Photophobia |
10 (4.7) |
1 (0.5) |
12 (5.9) |
1 (0.5) |
10 (5.4) |
1 (0.5) |
|
Vital dye staining cornea present |
7 (3.3) |
5 (2.3) |
2 (1.0) |
1 (0.5) |
1 (0.5) |
0 |
|
Ocular hyperaemia |
6 (2.8) |
3 (1.4) |
4 (2.0) |
2 (1.0) |
4 (2.2) |
4 (2.2) |
|
Instillation site pain |
5 (2.4) |
12 (5.6) |
11 (5.4) |
13 (6.3) |
0 |
0 |
|
Dry eye |
4 (1.9) |
2 (0.9) |
2 (1.0) |
2 (1.0) |
9 (4.9) |
4 (2.2) |
|
Eye pain |
4 (1.9) |
5 (2.3) |
4 (2.0) |
2 (1.0) |
5 (2.7) |
6 (3.2) |
|
Headache |
4 (1.9) |
2 (0.9) |
3 (1.5) |
0 |
2 (1.1) |
2 (1.1) |
|
Visual impairment |
4 (1.9) |
0 |
2 (1.0) |
3 (1.5) |
2 (1.1) |
0 |
|
Eye irritation |
3 (1.4) |
8 (3.7) |
1 (0.5) |
1 (0.5) |
3 (1.6) |
2 (1.1) |
|
Vitreous detachment |
3 (1.4) |
1 (0.5) |
3 (1.5) |
1 (0.5) |
0 |
1 (0.5) |
|
Vitreous floaters |
3 (1.4) |
0 |
0 |
0 |
0 |
0 |
|
Bronchitis |
2 (0.9) |
0 |
2 (1.0) |
3 (1.5) |
0 |
0 |
|
Conjunctival haemorrhage |
2 (0.9) |
0 |
2 (1.0) |
3 (1.5) |
0 |
1 (0.5) |
|
Growth of eyelashes |
2 (0.9) |
3 (1.4) |
2 (1.0) |
5 (2.4) |
0 |
1 (0.5) |
|
Punctate keratitis |
2 (0.9) |
1 (0.5) |
2 (1.0) |
1 (0.5) |
1 (0.5) |
2 (1.1) |
|
Anterior chamber cell |
1 (0.5) |
1 (0.5) |
3 (1.5) |
0 |
0 |
0 |
|
Eyelid irritation |
1 (0.5) |
0 |
1 (0.5) |
0 |
2 (1.1) |
1 (0.5) |
|
Foreign body sensation in eyes |
1 (0.5) |
0 |
2 (1.0) |
0 |
1 (0.5) |
3 (1.6) |
|
Lacrimation increased |
1 (0.5) |
0 |
2 (1.0) |
0 |
2 (1.1) |
1 (0.5) |
|
Arthralgia |
0 |
1 (0.5) |
0 |
0 |
0 |
3 (1.6) |
|
Blepharal pigmentation |
0 |
1 (0.5) |
0 |
0 |
0 |
2 (1.1) |
|
Cataract |
0 |
0 |
0 |
0 |
0 |
2 (1.1) |
|
Conjunctival irritation |
0 |
0 |
0 |
0 |
3 (1.6) |
1 (0.5) |
|
Conjunctivitis |
0 |
0 |
1 (0.5) |
1 (0.5) |
3 (1.6) |
5 (2.7) |
|
Corneal pigmentation |
0 |
0 |
0 |
0 |
2 (1.1) |
0 |
|
Corneal thickening |
0 |
0 |
0 |
0 |
7 (3.8) |
2 (1.1) |
|
Eye discharge |
0 |
0 |
1 (0.5) |
0 |
2 (1.1) |
0 |
|
Eye pruritus |
0 |
1 (0.5) |
2 (1.0) |
0 |
2 (1.1) |
4 (2.2) |
|
Eyelids pruritus |
0 |
0 |
0 |
0 |
2 (1.1) |
0 |
|
Intraocular pressure increased |
0 |
0 |
5 (2.5) |
0 |
1 (0.5) |
0 |
|
Keratic precipitates |
0 |
0 |
0 |
0 |
3 (1.6) |
0 |
|
Nasopharyngitis |
0 |
4 (1.9) |
1 (0.5) |
0 |
1 (0.5) |
4 (2.2) |
|
Sinusitis |
0 |
1 (0.5) |
0 |
3 (1.5) |
0 |
0 |
|
Upper respiratory tract infection |
0 |
3 (1.4) |
5 (2.5) |
3 (1.5) |
0 |
1 (0.5) |
Source: Adapted from FDA Review
Abbreviations: AE, adverse event; CI, confidence interval; N, number of patients in treatment arm; n, number of patients with adverse event
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- *Race: The occurrence of side effects was similar in White and Black or African American patients.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
*In Trials 1 and 2, the number of patients of races other than White and Black was small; therefore, differences in how OMLONTI worked among races could not be determined. Trial 3 was exclusively conducted in Asia therefore analysis by race is not conducted for this study.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 14. Overview of Side Effects by Sex, Race, and Age in Trial 1 (011709IN), Safety Population, Double Masked Period
|
Characteristic |
OMLONTI N=211 |
Timolol N=215 |
||||
|
All Patients n (%) |
All Grades |
Grades 3 to 4 |
All Patients n (%) |
All Grades |
Grades 3 to 4 |
|
|
Sex |
|
|
|
|
|
|
|
Female |
122 (57.8) |
55/122 (45.1) |
5/122 (4.1) |
136 (63.3) |
44/136 (32.4) |
5/136 (3.7) |
|
Male |
89 (42.2) |
33/89 (37.1) |
1/89 (1.1) |
79 (36.7) |
33/79 (41.8) |
1/79 (1.3) |
|
Race |
|
|
|
|
|
|
|
White |
159 (75.4) |
79/159 (49.7) |
6/159 (3.8) |
157 (73.0) |
61/157 (38.9) |
5/157 (3.2) |
|
American Indian or Alaska Native |
0 (0) |
0/0 (NA) |
0/0 (NA) |
1 (0.5) |
0/1 (0) |
0/1 (0) |
|
Asian |
0 (0) |
0/0 (NA) |
0/0 (NA) |
2 (0.9) |
1/2 (50.0) |
0/2 (0) |
|
Black or African American |
48 (22.7) |
8/48 (16.7) |
0/48 (0) |
52 (24.2) |
12/52 (23.1) |
1/52 (1.9) |
|
Multiple |
2 (0.9) |
1/2 (50.0) |
0/2 (0) |
3 (1.4) |
3/3 (100) |
0/3 (0) |
|
Native Hawaiian or Other Pacific Islander |
2 (0.9) |
0/2 (0) |
0/2 (0) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
Age group, years |
|
|
|
|
|
|
|
<18 |
6 (2.8) |
0/6 (0) |
0/6 (0) |
7 (3.3) |
1/7 (14.3) |
0/7 (0) |
|
≥18 and <65 |
82 (38.9) |
34/82 (41.5) |
2/82 (2.4) |
93 (43.3) |
34/93 (36.6) |
2/93 (2.2) |
|
≥65 |
123 (58.3) |
54/123 (43.9) |
4/123 (3.3) |
115 (53.5) |
42/115 (36.5) |
4/115 (3.5) |
Source: Adapted from FDA Review
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; NA, not applicable; Ns, total number of patients in each category
Table 15. Overview of Side Effects by Sex, Race, and Age in Trial 2 (011710IN), Safety Population, Entire Study Period
|
Characteristic |
OMLONTI N=204 |
Timolol N=205 |
||||
|
All Patients n (%) |
All Grades |
Grades 3 to 4 |
All Patients n (%) |
All Grades |
Grades 3 to 4 |
|
|
Sex |
|
|
|
|
|
|
|
Female |
121 (59.3) |
50/121 (41.3) |
6/121 (5.0) |
109 (53.2) |
37/109 (33.9) |
0/109 (0) |
|
Male |
83 (40.7) |
34/83 (41.0) |
0/83 (0) |
96 (46.8) |
30/96 (31.2) |
1/96 (1.0) |
|
Race |
|
|
|
|
|
|
|
White |
122 (59.8) |
60/122 (49.2) |
6/122 (4.9) |
140 (68.3) |
52/140 (37.1) |
0/140 (0) |
|
American Indian or Alaska Native |
0 (0) |
0/0 (NA) |
0/0 (NA) |
1 (0.5) |
0/1 (0) |
0/1 (0) |
|
Asian |
8 (3.9) |
2/8 (25.0) |
0/8 (0) |
8 (3.9) |
2/8 (25.0) |
0/8 (0) |
|
Black or African American |
72 (35.3) |
22/72 (30.6) |
0/72 (0) |
54 (26.3) |
12/54 (22.2) |
1/54 (1.9) |
|
Multiple |
2 (1.0) |
0/2 (0) |
0/2 (0) |
1 (0.5) |
1/1 (100) |
0/1 (0) |
|
Native Hawaiian or Other Pacific Islander |
0 (0) |
0/0 (NA) |
0/0 (NA) |
1 (0.5) |
0/1 (0) |
0/1 (0) |
|
Age group, years |
|
|
|
|
|
|
|
<18 |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
≥18 and <65 |
89 (43.6) |
36/89 (40.4) |
2/89 (2.2) |
87 (42.4) |
24/87 (27.6) |
1/87 (1.1) |
|
≥65 |
115 (56.4) |
48/115 (41.7) |
4/115 (3.5) |
118 (57.6) |
43/118 (36.4) |
0/118 (0) |
Source: Adapted from FDA Review
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; NA, not applicable; Ns, total number of patients in each category
Table 16. Overview of Side Effects by Sex, Race, and Age in Trial 3 (01171505), Safety Population, Entire Study Period
|
Characteristic |
OMLONTI N=185 |
Latanoprost N=185 |
||||
|
All Patients n (%) |
All Grades |
Grades 3 to 4 |
All Patients n (%) |
All Grades |
Grades 3 to 4 |
|
|
Sex |
|
|
|
|
|
|
|
Female |
78 (42.2) |
30/78 (38.5) |
0/78 (0) |
97 (52.4) |
24/97 (24.7) |
2/97 (2.1) |
|
Male |
107 (57.8) |
44/107 (41.1) |
1/107 (0.9) |
88 (47.6) |
31/88 (35.2) |
0/88 (0) |
|
Race |
|
|
|
|
|
|
|
Asian |
185 (100) |
74/185 (40.0) |
1/185 (0.5) |
185 (100) |
55/185 (29.7) |
2/185 (1.1) |
|
American Indian or Alaska Native |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
Black or African American |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
Multiple |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
Native Hawaiian or Other Pacific Islander |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
White |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
Age group, years |
|
|
|
|
|
|
|
<18 |
0 (0) |
0/0 (NA) |
0/0 (NA) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
≥18 and <65 |
138 (74.6) |
55/138 (39.9) |
0/138 (0) |
143 (77.3) |
40/143 (28.0) |
1/143 (0.7) |
|
≥65 |
47 (25.4) |
19/47 (40.4) |
1/47 (2.1) |
42 (22.7) |
15/42 (35.7) |
1/42 (2.4) |
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION