Drug Trials Snapshot: SILIQ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to SILIQ Prescribing Information for complete information.
SILIQ (brodalumab)
(Sil-eek)
Valeant Pharmaceuticals
Approval date: February 15, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
SILIQ is used for treatment of moderate to severe plaque psoriasis in adults.
- who may benefit from systemic treatment (such as injections or pills) or phototherapy (ultraviolet light treatment) and
- who did not respond or lost response to other systemic treatments.
How is this drug used?
SILIQ is an injection given under the skin once every week for the first three injections followed by an injection once every two weeks.
What are the benefits of this drug?
Clinical trials showed that SILIQ was better than a placebo in improving symptoms of plaque psoriasis and maintaining the improvement through a year of treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trials based on the two co-primary endpoints: 1) PASI 75, the proportion of subjects who achieved at least a 75% reduction in the Psoriasis Area and Severity Index (PASI) composite score that takes into consideration both the percentage of body surface area affected and the nature and severity of psoriatic changes (induration, erythema, and scaling) within the affected region, and 2) the proportion of subjects with a static Physicians Global Assessment (sPGA) score of 0 (clear) or 1 (almost clear), and at least a 2-point improvement from baseline. In Trials 2 and 3, comparisons were also made to ustekinumab for the primary endpoint of the proportion of subjects who achieved a reduction in PASI score of 100% (PASI 100) from baseline at Week 12.
Results are presented using efficacy or ITT (Intend to Treat) population
Table 2. Efficacy Results at Week 12 in Adults with Plaque Psoriasis in Trials 1, 2, and 3; NRIa
| Endpoint | Trial 1 | Trial 2 | Trial 3 | |||||
|---|---|---|---|---|---|---|---|---|
| SILIQ (N=222) n (%) | Placebo (N=220) n (%) | SILIQ (N=612) n (%) | Ustekinumab (N=300) n (%) | Placebo (N=309) n (%) | SILIQ (N=624) n (%) | Ustekinumab (N=313) n (%) | Placebo (N=315) n (%) | |
| PASI 75b response | 185 (83) | 6 (3) | 528 (86) | 210 (70) | 25 (8) | 531 (85) | 217 (69) | 19 (6) |
| PASI 100 response | 93 (42) | 1 (> | 272 (44) a | 65 (22) | 2 (1) | 229 (37)a | 58 (19) | 1 (> |
| sPGA success clear (0) or almost clear (1)b | 168 (76) | 3 (1) | 481 (79) | 183 (61) | 12 (4) | 497 (80) | 179 (57) | 13 (4) |
| sPGA of clear (0) | 93 (42) | 1 (> | 274 (45) | 65 (21) | 2 (1) | 229 (37) | 58 (19) | 1 (> |
a NRI=non-responder imputation | ||||||||
PASI= Psoriasis Area and Severity Index
sPGA= static Physician Global Assessment
SILIQ Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: SILIQ worked similarly in men and women.
- Race: The majority of patients were white. The number of patients in other races was limited; therefore, differences in response among races could not be determined.
- Age: SILIQ worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by subgroup for each trial (Trials 1, 2 and 3) based on efficacy or ITT population.
Table 3. Success on the sPGA at Week 12 by Baseline Demographics for Trials 1, 2, and 3
| Trial 1 | Trial 2 | Trial 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| SILIQ N=222 x/n (%) | Placebo N=220 x/n (%) | SILIQ N=612 x/n (%) | Placebo N=309 x/n (%) | Ustekinumab N=300 x/n (%) | SILIQ N=624 x/n (%) | Placebo N=315 x/n (%) | Ustekinumab N=313 x/n (%) | |
| Sex | ||||||||
| Men | 120/161 (75) | 2/161 (1) | 330/421 (78) | 6/219 (3) | 120/205 (59) | 351/431 (81) | 8/208 (4) | 117/212 (55) |
| Women | 48/61 (79) | 1/59 (2) | 151/191 (79) | 6/90 (7) | 63/95 (66) | 146/193 (76) | 5/107 (5) | 62/101 (61) |
| Age Group | ||||||||
| > | 154/206 (75) | 3/202 (1) | 458/585 (78) | 11/289 (4) | 174/279 (62) | 467/578 (81) | 11/300 (4) | 168/292 (58) |
| ≥65 | 14/16 (88) | 0/18 (0%) | 23/27 (85) | 1/20 (5) | 9/21 (43) | 30/46 (65) | 2/15 (13) | 11/21 (52) |
| Race | ||||||||
| White | 152/203 (75) | 2/202 (1) | 435/551 (79) | 9/273 (3) | 166/271 (61) | 448/565 (79) | 13/294 (4) | 162/280 (58) |
| Non-white | 16/19 (84) | 1/18 (6) | 46/61 (75) | 3/36 (8) | 17/29 (59) | 49/59 (83) | 0/21 (0) | 17/33 (52) |
| Region | ||||||||
| U.S.A. | 60/87 (69) | 3/88 (3) | 209/286 (73) | 5/144 (3) | 79/140 (56) | 203/276 (74) | 7/141 (5) | 78/140 (56) |
| Canada | 40/48 (83) | 0/47 (0) | 53/64 (83) | 2/33 (6) | 23/32 (72) | 59/72 (82) | 0/34 (0) | 23/38 (61) |
| Ex-North America | 68/87 (78) | 0/85 (0) | 219/262 (84) | 5/132 (4) | 81/128 (63) | 235/276 (85) | 6/140 (4) | 78/135 (58) |
Adapted from FDA Statistical review
What are the possible side effects?
SILIQ may cause serious side effects including suicidal thoughts and behavior, infections, and “flare-ups” of Crohn’s disease. Because of a suicide risk, SILIQ is available only through a restricted program called the SILIQ REMS Program.
The most common side effects of SILIQ are joint pains, headache, tiredness, diarrhea, throat pain, nausea, muscle pain, injection site reactions, flu, low white blood cell count and fungal infections of the skin.
Before starting SILIQ, patients should be evaluated for tuberculosis infection.
What are the possible side effects?
Suicidal ideation and behavior, including completed suicides, have occurred in patients treated with SILIQ. Patients and caregivers should seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety, or other mood changes. Patients should carry the wallet card provided and call the National Suicide Prevention Lifeline at 1-800-273-8255 if they experience suicidal thoughts.
SILIQ may cause serious infections. Before starting SILIQ, patients should be evaluated for tuberculosis infection.
Patients with Crohn’s disease should not use SILIQ.
The table below summarizes the most common, less severe adverse reactions that occurred in clinical trials. The results are based on safety population defined as all patients in the trials who received at least one dose of SILIQ and patients who remained on placebo or ustekinumab by the end of the trial.
Table 4. Adverse Reactions Occurring in ≥ 1% of Subjects in the SILIQ Group and More Frequently than in the Placebo Group in Plaque Psoriasis Trials through Week 12
| Adverse Reactions | Placebo (N=879) n (%) | SILIQ a (N=1496) n (%) | Ustekinumab (N=613)b n (%) |
|---|---|---|---|
| Arthralgia | 29 (3.3) | 71 (4.7) | 15 (2.4) |
| Headache | 31 (3.5) | 64 (4.3) | 23 (3.8) |
| Fatigue | 10 (1.1) | 39 (2.6) | 16 (2.6) |
| Diarrhea | 10 (1.1) | 33 (2.2) | 5 (0.8) |
| Oropharyngeal pain | 10 (1.1) | 31 (2.1) | 8 (1.3) |
| Nausea | 10 (1.1) | 28 (1.9) | 6 (1.0) |
| Myalgia | 3 (0.3) | 26 (1.7) | 4 (0.7) |
| Injection site reactions (pain, erythema, bruising, hemorrhage, pruritus) | 11 (1.3) | 23 (1.5) | 12 (2.0) |
| Influenza | 4 (0.5) | 19 (1.3) | 7 (1.1) |
| Neutropenia | 4 (0.5) | 15 (1.0) | 5 (0.8) |
| Tinea infections (tinea pedis, versicolor, cruris) | 2 (0.2) | 15 (1.0) | 3 (0.5) |
a subjects receiving 210 mg of SILIQ at Weeks 0, 1, and 2, followed by treatment every two weeks during the 12-week period
b Trials 2 and 3 included the active comparator, ustekinumab.
SILIQ Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in men and women.
- Race: The majority of patients were white. The number of patients in other races was limited; therefore, differences in side effects among races could not be determined.
- Age: The risk of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes treatment emergent adverse events (TEAEs) by subgroup for the pooled safety population from Trials 1, 2, and 3.
Table 5. Subgroup Analysis of TEAEs (safety population)
| Subgroup | Placebo (N=879) x/n* (%) | SILIQ (N=1496) x/n* (%) | Ustekinumab (N=613) x/n* (%) |
|---|---|---|---|
| Sex | |||
| Men | 297/607 (49) | 580/1037 (56) | 225/417 (54) |
| Women | 154/272 (57) | 290/459 (63) | 120/196 (61) |
| Age Group | |||
| 65> | 423/823 (51) | 828/1406 (59) | 325/571 (57) |
| ≥65 years | 28/56 (50) | 42/90 (47) | 20/42 (47) |
| Race | |||
| White | 413/799 (52) | 793/1351 (59) | 316/551 (57) |
| Black or African American | 14/29 (48) | 18/40 (45) | 9/20 (45) |
| Asian | 12/29 (41) | 27/51 (53) | 11/24 (46) |
| All other races combined | 12/22 (55) | 12/54 (22) | 9/18 (50) |
**x/n= number of patents with adverse reaction (x) in the subgroup (n)
Adapted from Clinical Study Report
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved SILIQ based on evidence mainly from three clinical trials of 2915 patients with moderate to severe psoriasis. The trials were conducted in the USA, Canada, Europe, and Australia.
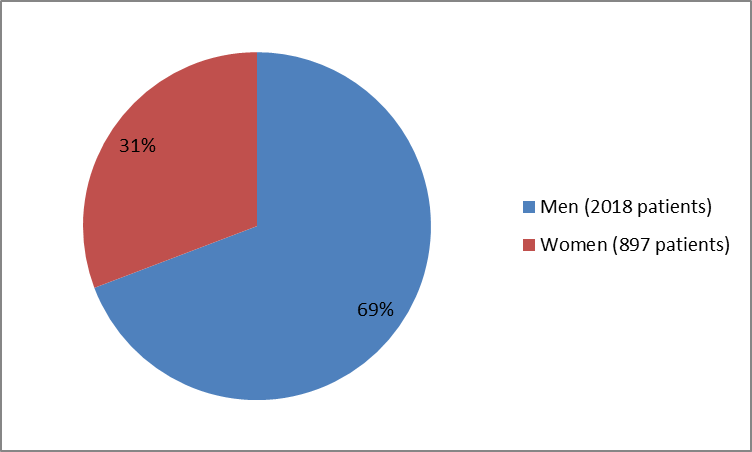
The figure below summarizes how many men and women were in the clinical trials used to evaluate the benefit of the drug. This population is called the efficacy population.
Figure 1. Baseline Demographics by Sex (efficacy population)
Adapted from FDA Statistical review
Figure 2 and Table 1 below summarize the percentage of patients by race based on efficacy population.
Figure 2. Baseline Demographics by Race (efficacy population)
Adapted from FDA Statistical review
Table 1. Baseline Demographics by Race (efficacy population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2693 | 91% |
| Asian | 102 | 3% |
| Black or African American | 85 | 3% |
| America Indian or Alaska Native | 11 | less than 1% |
| Native Hawaiian or Other Pacific Islander | 14 | less than 1% |
| Multiple | 11 | less than 1% |
| Other | 53 | 2% |
Adapted from FDA Statistical review
Figure 3 summarizes the percentage of patients by age based on the efficacy population.
Figure 3. Baseline Demographics by Age (efficacy population)
Adapted from FDA Statistical review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials
Table 6. Baseline Demographics of Patients in the Clinical Trials (efficacy population)
| Trial 1 | Trial 2 | Trial 3 | Total N=2915 n(%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SILIQ N=222 n(%) | Placebo N=22 n(%) | SILIQ N=612 n(%) | Placebo N=309 n(%) | Ustekinumab N=300 n(%) | SILIQ N=624 n(%) | Placebo N=315 n(%) | Ustekinumab N=313 n(%) | ||||
| Sex | |||||||||||
| Men | 161 (73) | 161 (73) | 421 (69) | 219 (71) | 205 (68) | 431 (69) | 208 (66) | 212 (68) | 2018 (69) | ||
| Women | 61 (28) | 59 (27) | 191 (31) | 90 (29) | 95 (32) | 193 (31) | 107 (34) | 101 (32) | 897 (31) | ||
| Age | |||||||||||
| Mean | 46 | 47 | 45 | 44 | 45 | 45 | 44 | 45 | 45 | ||
| SD | 12 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | ||
| Range | 20-75 | 20-86 | 18-75 | 18-76 | 18-75 | 18-75 | 18-75 | 18-74 | 18-86 | ||
| Age Group | |||||||||||
| 65> | 206 (93) | 202 (92) | 585 (96) | 289 (94) | 279 (93) | 578 (93) | 300 (95) | 292 (93) | 2731 (94) | ||
| ≥65 years | 16 (7) | 18 (8) | 27 (4) | 20 (7) | 21 (7) | 46 (7) | 15 (5) | 21 (7) | 184 (6) | ||
| Race | |||||||||||
| White | 203 (91) | 202 (92) | 551 (90) | 273 (88) | 271 (90) | 565 (91) | 294 (93) | 280 (90) | 263 (91) | ||
| Asian | 10 (5) | 8 (4) | 19 (3) | 12 (4) | 12 (4) | 20 (3) | 9 (3) | 12 (4) | 102 (3) | ||
| Black or African American | 3 (1) | 6 (3) | 19 (3) | 14 (5) | 7 (2) | 17 (3) | 6 (2) | 13 (4) | 85 (3) | ||
| American Indian or Alaska Native | 1 (> | 0 (0) | 3 (> | 2 (> | 1 (> | 3 (> | 0 (0) | 1 (> | 11 (> | ||
| Native Hawaiian or Other Pacific Islander | 2 (1) | 2 (1) | 5 (1) | 0 (0) | 0 (0) | 3 (> | 1 (> | 1 (> | 14 (> | ||
| Multiple | 0 (0) | 0 | 5 (1) | 1 (> | 3 (1) | 2 (> | 0 (0) | 0 (0) | 11 (> | ||
| Other | 3 (1) | 2 (1) | 10 (2) | 7 (2) | 6 (2) | 14 (2) | 5 (2) | 6 (2) | 53 (2) | ||
Adapted from FDA Statistical review
How were the trials designed?
The benefit and side effects of SILIQ were evaluated in three clinical trials of patients with moderate to severe psoriasis. In all three trials patients received treatment with either SILIQ or placebo. Some patients in Trials 2 and 3 also received drug approved for psoriasis called ustekinumab. Neither the patients nor the health care providers knew which treatment was being given until after the first 12 weeks of the trials were completed.
Patients were evaluated for improvement of psoriasis after 12 weeks of treatment by scoring of the extent, nature and severity of psoriatic changes of the skin. Some patients continued on the treatment for 12 months to determine how long the benefit would last.
How were the trials designed?
The safety and efficacy of SILIQ were established in 3 randomized, double-blind, placebo-controlled trials (Trials 1, 2 and 3) of 12 weeks duration. In the two placebo and active comparator trials (Trials 2 and 3), subjects were also randomized to receive U.S. approved ustekinumab for 12 weeks.
All patients had moderate to severe plaque psoriasis defined as a minimum body surface area involvement of 10%, a static Physician Global Assessment (sPGA) score of ≥3, a Psoriasis Area and Severity Index (PASI) score ≥12, and were candidates for phototherapy or systemic therapy. Approximately 30% of patients had previously received a biologic therapy and 12% of patients had failed previous biologic therapy.
All three trials assessed the change from baseline to Week 12 compared to placebo in the two co-primary endpoints: PASI 75 (the proportion of subjects who achieved at least a 75% reduction in the PASI composite score) and sPGA of “0” (clear) or “1” (minimal) with at least a 2-point improvement from the baseline. In Trials 2 and 3, comparisons were also made to ustekinumab for the primary endpoint of the proportion of subjects who achieved a reduction in PASI score of 100% (PASI 100) from baseline at Week 12.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.