Drug Trials Snapshot: TRYNGOLZA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the TRYNGOLZA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
TRYNGOLZA (olezarsen) injection
trin-GOLE-zah
Ionis Pharmaceuticals Inc.
Original Approval date: December 19, 2024
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TRYNGOLZA is a prescription medicine (an APOC-III-directed antisense oligonucleotide) that is used along with a low-fat diet to reduce triglycerides in adults with familial chylomicronemia syndrome (FCS).
FCS is a rare genetic disorder that prevents the body from breaking down fats.
How is this drug used?
TRYNGOLZA is a subcutaneous injection that is taken once every month.
Who participated in the clinical trials?
The FDA approved TRYNGOLZA based on evidence from a clinical trial (Trial 1; NCT04568434) of 66 patients with FCS. The trial was conducted at 29 sites in 11 countries including Canada, France, Italy, Netherlands, Norway, Portugal, Slovakia, Spain, Sweden, the United Kingdom, and the United States. Of the 66 patients, 19 patients were from trial sites in the United States.
The benefits and side effects of TRYNGOLZA for patients with FCS were evaluated in the same single clinical trial. Additional trials in patients with hypertriglyceridemia were used to support the safety assessment. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
How were the trials designed?
The benefits and side effects of TRYNGOLZA were evaluated in one clinical trial of 66 participants with FCS.
Enrolled participants were already using other treatments to lower triglycerides, including a low-fat diet and medications (such as fenofibrates, omega-3 fatty acids, and statins). Participants were randomly assigned to receive TRYNGOLZA or placebo every four weeks for one year. Neither the participants nor the health care providers knew which treatment was being given.
The trial measured percent change in triglycerides from baseline (before treatment) to Month 6 and compared TRYNGOLZA to placebo.
How were the trials designed?
The efficacy of TRYNGOLZA was demonstrated in a randomized, placebo-controlled, double-blind clinical trial (Trial 1; NCT04568434) in adult patients with genetically diagnosed FCS and fasting triglyceride levels ≥880 mg/dL. After a ≥4-week run-in period where patients continued to follow a diet with ≤20 grams of fat per day, patients were randomly assigned to receive doses every four weeks of TRYNGOLZA 80 mg (n=22) or matching volume of placebo (n=23) via subcutaneous injection over a 53-week treatment period. The primary endpoint was percent change in fasting triglycerides from baseline to Month 6 (average of Weeks 23, 25, and 27) compared to placebo.
DEMOGRAPHICS SNAPSHOT
Figure 1 summarizes Figure 1 summarizes how many male and female patients were enrolled in the trial used to evaluate the efficacy of TRYNGOLZA.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
Figure 2 summarizes how many patients by race were enrolled in the trial used to evaluate the efficacy of TRYNGOLZA.
Figure 2. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
Figure 3 summarizes how many patients by age were enrolled in the trial used to evaluate the efficacy of TRYNGOLZA.
Figure 3. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
Figure 4 summarizes how many patients by ethnicity were enrolled in the trial used to evaluate the efficacy of TRYNGOLZA.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics
| Baseline Demographics | TRYNGOLZA 50 mg* N=21 | TRYNGOLZA 80 mg N=22 | Placebo N=23 |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 15 (71.4) | 11 (50.0) | 12 (52.2) |
| Male | 6 (28.6) | 11 (50.0) | 11 (47.8) |
| Age, years | |||
| Mean (SD) | 43.2 (12.1) | 47.7 (13.3) | 44.0 (14.7) |
| Minimum, maximum | 18, 74 | 25, 78 | 21, 69 |
| Age category, years, n (%) | |||
| <65 | 20 (95.2) | 20 (90.9) | 20 (87.0) |
| ≥65 | 1 (4.8) | 2 (9.1) | 3 (13.0) |
| Race, n (%) | |||
| White | 17 (81.0) | 17 (77.3) | 22 (95.7) |
| Asian | 3 (14.3) | 3 (13.6) | 0 |
| Native Hawaiian or other Pacific Islander | 1 (4.8) | 0 | 0 |
| Other | 0 | 2 (9.1) | 1 (4.3) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 3 (14.3) | 1 (4.5) | 3 (13.0) |
| Not Hispanic or Latino | 18 (85.7) | 21 (95.5) | 20 (87.0) |
Source: Adapted from FDA Review
* TRYNGOLZA 50 mg is not an approved dosing regimen
Abbreviations: SD, standard deviation
What are the benefits of this drug?
In the clinical trial, participants who received TRYNGOLZA had a 42.5% reduction in triglycerides compared to participants who received placebo after six months of treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2. Percent Change in Triglycerides After 6 Months of Treatment in Participants With FCS on Other Triglyceride-Lowering Therapies in Trial 1, Efficacy Population
| Parameter | TRYNGOLZA 80 mg | Placebo | Treatment Difference (95% CI) |
|---|---|---|---|
| Mean percent change in triglycerides | -30% | +12% | -42.5% (-74, -11) |
Source: TRYNGOLZA Prescribing Information
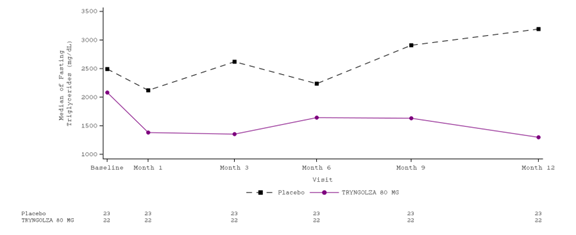
Figure 5 shows the absolute change in triglycerides over time.
Figure 5. Fasting Triglycerides (mg/dL) Over Time in Trial 1, Efficacy Population
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: The observed effect of TRYNGOLZA was similar for females and males.
- Race: The number of patients of races other than White was small; therefore, differences in how TRYNGOLZA worked among races could not be determined.
- Age: Because almost all adult participants were younger than 65 years of age, differences between age groups in how TRYNGOLZA worked could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3. Percent Change in Baseline in Fasting Triglycerides (mg/dL) at Month 6 by Subgroup, TRYNGOLZA 80 mg vs. Placebo, Efficacy Population
| Group | Subgroup | LS Mean | 95% Lower CI | 95% Upper CI |
|---|---|---|---|---|
| Sex | Female | -31.1 | -55.6 | -8.9 |
| Male | -31.8 | -56.2 | -7.8 | |
| Age, Years | <65 | -23.7 | -48.0 | -7.6 |
| ≥65 | -18.3 | -28.2 | -8.6 | |
| Race | Other | -38.9 | -94.0 | 0.2 |
| White | -35.3 | -58.4 | -13.6 |
Source: FDA Statistical Review
Abbreviations: CI, credible interval; LS, least squares
What are the possible side effects?
Most common side effects include injection site reactions, low platelet counts, and joint pain.
Allergic (hypersensitivity) reactions, including difficulty breathing, rash, facial swelling, hives, chills, and muscle aches have been reported in some patients taking TRYNGOLZA.
What are the possible side effects (results of trials used to assess safety)?
Table 4. Adverse Reactions Occurring >5% of Patients With FCS Treated With TRYNGOLZA and at >3% Higher Frequency Than Placebo, Safety Population
| Adverse Reactiona | Total TRYNGOLZA N=43 n (%) | Placebo N=23 n (%) |
|---|---|---|
| Injection site reactions | 8 (19) | 2 (9) |
| Thrombocytopenia | 5 (12) | 1 (4) |
| Arthralgia | 4 (9) | 0 |
Source: TRYNGOLZA Prescribing Information
a Grouped terms composed of several similar terms
Abbreviations: FCS, familial chylomicronemia syndrome
The laboratory measures and the direction of change associated with TRYNGOLZA treatment include:
- Platelet counts may decrease
- Glucose levels may increase
- Liver enzymes may increase
- Low-density lipoprotein cholesterol (LDL-C) may increase
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in females and males.
- Race: The number of patients of races other than White was small; therefore, differences in side effects among other races could not be determined.
- Age: The number of patients older than 65 years was low, therefore, differences in side effects by age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
There were no substantial differences in the risk of treatment-emergent adverse events (TEAEs) in any subgroup. However, the overall safety database and individual subgroup sample sizes were not sufficient to detect clinically meaningful differences in the frequency of TEAEs between individual subgroups.
Table 5. Side Effects by Subgroup, Safety Population
| Characteristic | TRYNGOLZA 80 mg N=22 n/Ns (%) | Placebo N=23 n/Ns (%) |
|---|---|---|
| Sex | ||
| Female | 11/11 (100) | 10/12 (83.3) |
| Male | 8/11 (72.7) | 11/11 (100) |
| Age group, years | ||
| <40 | 6/7 (85.7) | 8/9 (88.9) |
| ≥40 to <65 | 11/13 (84.6) | 11/11 (100) |
| ≥65 | 2/2 (100) | 2/3 (66.7) |
| Age group ≥75, years | ||
| ≥75 | 1/1 (100) | 0/0 (NA) |
| Race | ||
| Asian | 3/3 (100) | 0/0 (NA) |
| Native Hawaiian or other Pacific Islander | 0/0 (NA) | 0/0 (NA) |
| Other | 1/2 (50.0) | 1/1 (100) |
| White | 15/17 (88.2) | 20/22 (90.9) |
| Ethnicity | ||
| Hispanic or Latino | 1/1 (100) | 3/3 (100) |
| Not Hispanic or Latino | 18/21 (85.7) | 18/20 (90.0) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; N, number of patients in treatment arm; n, number of patients with adverse event; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.