Drug Trials Snapshot: VERQUVO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the VERQUVO Prescribing Information for complete information.
VERQUVO (vericiguat)
(ver-KYU-voh)
Merck Sharp & Dohme Corp.
Approval date: January 19, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VERQUVO is a drug used to reduce the risk of dying and hospitalization in patients with chronic (long-lasting) heart failure.
Heart failure is a condition when the heart is not pumping as well as it should be.
How is this drug used?
VERQUVO is a tablet that is taken by mouth once a day.
VERQUVO is used in patients who are having symptoms of chronic heart failure and who have had a recent hospitalization or the need to receive intravenous medicines.
What are the benefits of this drug?
VERQUVO reduces the risk of dying and hospitalization in patients with chronic heart failure.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of VERQUVO is summarized in the table below. On average, for every 100 patients who took VERQUVO for one year, VERQUVO reduced the number of cardiovascular deaths or heart failure hospitalizations by 4.2: 1.0 cardiovascular deaths and 3.2 hospitalizations for heart failure.
Table 1. Primary Composite Endpoint and Secondary Endpoints of Cardiovascular Death and Heart Failure Hospitalization: Numbers of Events per 100 Patients per Year
|
|
VERQUVO |
Placebo |
Treatment Comparison |
|---|---|---|---|
|
|
Annual number of events per 100 patients |
Annual number of events per 100 patients |
Reduction in annual events per 100 patients |
|
Primary endpoint |
|||
|
Composite of |
33.6 |
37.8 |
4.2* |
|
Secondary endpoints |
|||
|
Cardiovascular |
12.9 |
13.9 |
1.0 |
|
Heart failure |
25.9 |
29.1 |
3.2 |
* The p-value was < 0.05, indicating statistical significance.
Source: Adapted from VERQUVO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: VERQUVO worked similarly in men and women.
- Race: VERQUVO worked similarly in Whites, Black or African Americans and Asians.
- Age: VERQUVO worked similarly in patients across tested age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
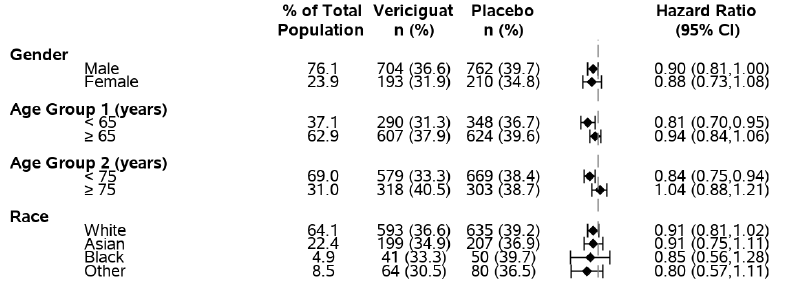
The figure below summarizes the results for the primary endpoint for subgroups based on sex, age and race. Each diamond (♦) represents the effect of VERQUVO as a hazard ratio. The vertical line indicates a hazard ratio of 1, the ratio at which there is no treatment difference between VERQUVO and placebo. The area to the left of the line represents a favorable treatment effect. The figure shows that treatment effects were similar across subgroups of sex, age and race.
Figure 5. Subgroup Analysis of the Primary Composite Endpoint
VERQUVO Prescribing Information
What are the possible side effects?
VERQUVO causes a harm to unborn baby and should not be given to pregnant women. Common side effects of VERQUVO are low blood pressure and low red cell count (anemia).
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes the most common side effects in the trial.
Table 2. Adverse Drug Reactions Occurring with VERQUVO in the Trial
|
Adverse Drug Reaction |
VERQUVO % |
Placebo % |
|---|---|---|
|
Hypotension |
16 |
15 |
|
Anemia |
10 |
7 |
VERQUVO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in men and women.
- Race: The risk of side effects was similar in Whites, Black or African Americans, and Asians.
- Age: The risk of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarize adverse reactions (hypotension and anemia) by subgroups.
Table 3. Most Common Adverse Events by Sex, Race and Age (Safety population): Percent of Patients
|
|
% of All |
Hypotension |
Anemia |
||
|---|---|---|---|---|---|
|
VERQUVO |
Placebo |
VERQUVO |
Placebo |
||
|
Sex |
|||||
|
Female |
24.0 |
15.1 |
14 |
9.4 |
7.5 |
|
Male |
76.0 |
16.8 |
15.2 |
9.7 |
7.3 |
|
Race |
|||||
|
Asian |
22.5 |
15.8 |
14.1 |
7.7 |
5.7 |
|
Black |
4.9 |
15.4 |
19 |
8.9 |
4.8 |
|
Multi-Racial |
7.2 |
19.1 |
21.1 |
15.3 |
10 |
|
White |
64 |
16.4 |
14.5 |
9.8 |
7.8 |
|
Age Group |
|||||
|
< 65 years |
37.1 |
17.1 |
12.6 |
7.3 |
5.4 |
|
≥65 years |
62.9 |
15.9 |
16.3 |
11 |
8.5 |
Source: Analysis provided by Division of Cardiology and Nephrology
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved VERQUVO based on evidence from a clinical trial (NCT02861534) which consisted of 5,050 patients 23 to 98 years old with worsening heart failure. The trial was conducted at 694 sites in 42 countries in Europe, Asia, North and South America.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex (Efficacy Population)
Adapted from FDA Review
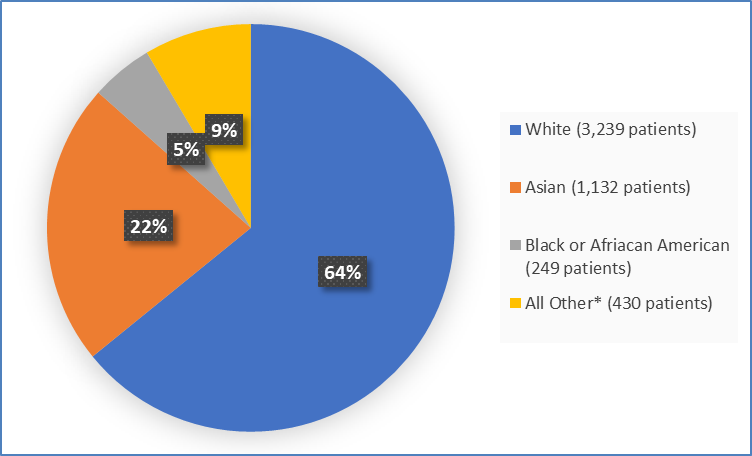
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race (Efficacy Population)
*includes Multiple, American Indian or Alaska Native, Native Hawaiian or Pacific Islander and Not Reported
Adapted from FDA Review
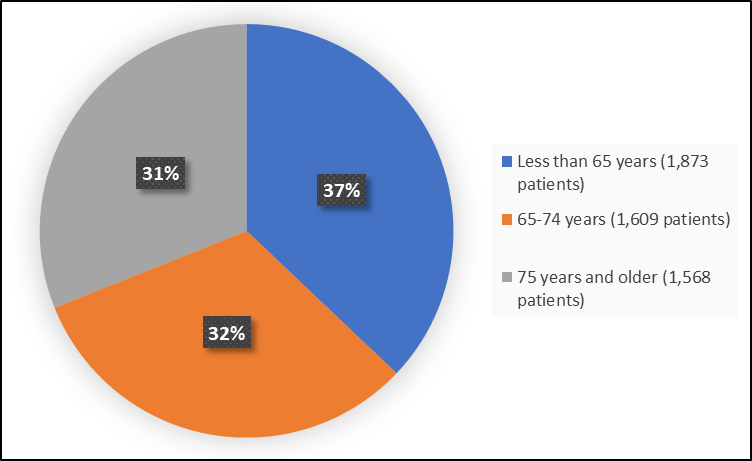
Figure 3 summarizes how many patients by age in the clinical trial.
Figure 3. Demographics by Age (Efficacy Population)
Adapted from FDA Review
Figure 4 summarizes how many patients by ethnicity in the clinical trial.
Figure 4. Demographics by Ethnicity (Efficacy Population)
Adapted from FDA Review
Who participated in the trials?
The table below summarizes demographics for the trial.
Table 4. Trial Demographics (Efficacy Population)
|
Demographic Category |
VERQUVO |
Placebo |
TOTAL |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Men |
1,921 (76.0) |
1,921 (76.1) |
3,842 (76.1) |
|
Women |
605 (24.0) |
603 (23.9) |
1,208 (23.9) |
|
Race, n (%) |
|||
|
White |
1,621 (64.2) |
1,618 (64.1) |
3,239 (64.1) |
|
Asian |
571 (22.6) |
561 (22.2) |
1,132 (22.4) |
|
Multiple |
183 (7.2) |
180 (7.1) |
363 (7.2) |
|
Black or African American |
123 (4.9) |
126 (5.0) |
249 (4.9) |
|
American Indian or Alaska Native |
24 (1.0) |
28 (1.1) |
52 (1.0) |
|
Native Hawaiian or Pacific Islander |
3 (0.1) |
11 (0.4) |
14 (0.3) |
|
Not Reported |
1 (0.04) |
0 |
1 (0.02) |
|
Age (years) |
|||
|
Median (Min, Max) |
69.0 (24, 98) |
68.0 (23, 97) |
69.0 (23, 98) |
|
Age Group (years) n (%) |
|||
|
<65 |
926 (36.6) |
947 (37.5) |
1,873 (37.1) |
|
≥65 |
1,600 (63.4) |
1,577 (63.5) |
3,177 (62.9) |
|
65-74 |
815 (32.3) |
794 (31.4) |
1,609 (31.9) |
|
≥75 |
785 (31.1) |
783 (31.0) |
1,568 (31.0) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
410 (16.2) |
403 (16.0) |
813 (16.1) |
|
Not Hispanic or Latino |
2,044 (80.9) |
2,065 (81.8) |
4,109 (81.4) |
|
Not Reported |
72 (2.9) |
56 (2.2) |
128 (2.5) |
|
Region,1 n (%) |
|||
|
Eastern Europe |
848 (33.6) |
846 (33.5) |
1694 (33.5) |
|
Asia Pacific |
592 (23.4) |
591 (23.4) |
1183 (23.4) |
|
Western Europe |
443 (17.5) |
446 (17.7) |
889 (17.6) |
|
Latin and South America |
362 (14.3) |
362 (14.3) |
724 (14.3) |
|
North America |
281 (11.1) |
279 (11.1) |
560 (11.1) |
|
United States |
208 (8.2) |
207 (8.2) |
415 (8.2) |
1 Eastern Europe: Czech Republic, Greece, Hungary, Israel, Poland, Russian Federation, South Africa, Turkey, Ukraine; Asia Pacific: Australia, China, Hong Kong, Japan, Korea, Malaysia, New Zealand, Philippines, Singapore, Taiwan; Western Europe: Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, Netherlands, Norway, Spain, Sweden, Switzerland, United Kingdom; Latin and South America: Argentina, Chile, Colombia, Guatemala, Mexico, Peru, Puerto Rico; North America: Canada, United States
Adapted from FDA Review
How were the trials designed?
The trial enrolled patients with symptoms of worsening heart failure. Patients were randomly assigned to receive VERQUVO or a placebo pill once a day. Neither the patients nor the health care professionals knew if the patients were given VERQUVO or placebo pill until after the trial was complete.
The trial measured the timing of two possible events:
- death of a patient that was considered to be related to diseases of blood vessels and heart, or
- the first time a patient needed to be hospitalized for worsening heart failure.
How were the trials designed?
The trial was a randomized, double-blind, multi-center trial comparing VERQUVO and placebo. The trial included adult patients with symptomatic New York Heart Association Class II to IV heart failure and left ventricular ejection fraction ≤ 45%. Patients were enrolled following a worsening heart failure event, defined as a heart failure hospitalization within 6 months before randomization or use of outpatient IV diuretics for heart failure within 3 months before randomization.
Patients were treated up to the target maintenance dose of VERQUVO 10 mg once daily or matching placebo. Therapy was initiated at VERQUVO 2.5 mg once daily and increased in approximately 2-week intervals to 5 mg once daily and then 10 mg once daily, as tolerated. After approximately 1 year, 90% of patients in both the VERQUVO and placebo arms were treated with the 10 mg target dose.
The primary endpoint was the time to first event of the composite of cardiovascular death or hospitalization for heart failure. The median follow-up for the primary endpoint was 11 months.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.