Drug Trials Snapshots: ADUHELM
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ADUHELM Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ADUHELM (Aducanumab)
(AD-yew-helm)
Biogen Inc.
Approval date: July 7, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ADUHELM is a prescription medicine used to treat people with Alzheimer’s disease. Alzheimer’s disease is a common degenerative disease of the brain that starts with mild thinking, judging, and memory problems and progresses to dementia and death. Treatment with ADUHELM should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials. There are no safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied.
How is this drug used?
ADUHELM is administered as an intravenous (IV) infusion over approximately one hour every four weeks and at least 21 days apart.
Who participated in the clinical trials?
The FDA approved ADUHELM primarily based on evidence from two large clinical trials (Trial 1/NCT 02484547 and Trial 2/NCT 02477800) of 3,285 patients with Alzheimer’s disease. The trials were conducted at over 300 sites in 19 countries globally. The effects of ADUHELM were also supported by a smaller trial (Trial 3/NCT 01677572) of 196 patients with Alzheimer’s disease conducted at 27 sites in the United States. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
Who participated in the trials?
Demographics of the population that participated in the ADUHELM trials are presented in Table 1 below.
Table 1. Trial Demographics
| Demographic Parameters | Trial 1 N=1638 n (%) |
Trial 2 N=1647 n (%) |
Trial 3 N=196 n (%) |
|---|---|---|---|
| Sex | |||
| Male | 795 (48.5) | 784 (47.6) | 98 (50) |
| Female | 843 (51.5) | 863 (52.4) | 98 (50) |
| Age | |||
| Mean years (SD) | 70.7 (7.4) | 70.1 (7.5) | 72.8 (7.9) |
| Median (years) | 72.0 | 71.0 | 73.0 |
| Min, max (years) | 50, 85 | 50, 85 | 51, 91 |
| Age group | |||
| ≤64 years | 323 (19.7) | 370 (22.5) | 31 (15.8) |
| >64 to <75 years | 774 (47.3) | 765 (46.4) | 84 (42.9) |
| ≥75 years | 541 (33.0) | 512 (31.1) | 81 (41.3) |
| Race | |||
| White | 1285 (78.4) | 1238 (75.2) | 191 (97) |
| Black or African American | 11 (0.7) | 8 (0.5) | 2 (1) |
| Asian | 128 (7.8) | 175 (10.6) | 1 (<1) |
| American Indian or Alaska Native | 1 (<0.1) | 0 | 1 (<1) |
| Native Hawaiian or Other Pacific Islander | 0 | 1 (<0.1) | 0 |
| Other | 5 (0.3) | 10 (0.6) | 1 (<1) |
| Not reported/Unknown | 208 (12.7) | 215 (13.1) | 0 |
| Ethnicity | |||
| Hispanic or Latino | 67 (4.1) | 37 (2.2) | 6 (3) |
| Not Hispanic or Latino | 1401 (85.5) | 1480 (89.9) | 190 (97) |
| Not reported | 169 (10.3) | 130 (7.9) | 0 |
| Region | |||
| United States | 652 (39.8) | 763 (46.3) | 196 (100) |
| Europe/Canada | 865 (52.8) | 721 (43.8) | 0 |
| Asia | 121 (7.4) | 163 (9.9) | 0 |
What are the benefits of this drug?
ADUHELM reduces the level of amyloid beta plaque in the brain of treated patients. It is believed that this reduction may predict clinical benefit in patients.
ADUHELM was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
More trials are ongoing to assess whether there is a clinical benefit of ADUHELM.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 presents amyloid beta positron emission tomography (PET) and cerebral spinal fluid (CSF) biomarker results from Trial 1. Table 3 presents results for clinical endpoints from Trial 1.
| Biomarker Endpoint at Week 781 | ADUHELM High Dose |
Placebo | |
|---|---|---|---|
| Amyloid beta PET composite SUVR | N=170 | N=159 | |
| Mean baseline | 1.383 | 1.375 | |
| Change from baseline | -0.264 | 0.014 | |
| Difference from placebo | -0.278, p<0.0001 | ||
| Amyloid beta PET centiloid | N=170 | N=159 | |
| Mean baseline | 85.3 | 83.5 | |
| Change from baseline (%) | -60.8 (-71%) | 3.4 | |
| Difference from placebo | -64.2, p<0.0001 | ||
| CSF p-tau (pg/mL) | N=17 | N=28 | |
| Mean baseline | 100.11 | 72.55 | |
| Change from baseline | -22.93 | -0.49 | |
| Difference from placebo | -22.44, p=0.0005 | ||
| CSF t-tau (pg/mL) | N=17 | N=28 | |
| Mean baseline | 686.65 | 484.00 | |
| Change from baseline | -112.44 | -0.39 | |
| Difference from placebo | -112.05, p=0.0088 | ||
Source: Adapted from FDA Review
1 P-values were not statistically controlled for multiple comparisons.
Abbreviations: CSF, cerebral spinal fluid; PET, positron emission tomography; p-tau, phosphorylated tau protein; SUVR, standard uptake value ratio; t-tau, total tau protein
Table 3. Clinical Results of ADUHELM in Trial 1
| Clinical Endpoint at Week 78 | ADUHELM High Dose N=547 |
Placebo N=548 |
|
|---|---|---|---|
| CDR-SB | |||
| Mean baseline | 2.51 | 2.47 | |
| Change from baseline | 1.35 | 1.74 | |
| Difference from placebo (%) | -0.39 (-22%), p=0.0120 | ||
| MMSE | |||
| Mean baseline | 26.3 | 26.4 | |
| Change from baseline | -2.7 | -3.3 | |
| Difference from placebo (%) | 0.6 (-18%), p=0.0493 | ||
| ADAS-Cog 13 | |||
| Mean baseline | 22.246 | 21.867 | |
| Change from baseline | 3.763 | 5.162 | |
| Difference from placebo (%) | -1.400 (-27%), p=0.0097 | ||
| ADCS-ADL-MCI | |||
| Mean baseline | 42.5 | 42.6 | |
| Change from baseline | -2.5 | -4.3 | |
| Difference from placebo (%) | 1.7 (-40%), p=0.0006 | ||
| NPI-101 | |||
| Mean baseline | 4.5 | 4.3 | |
| Change from baseline | 0.2 | 1.5 | |
| Difference from placebo (%) | -1.3 (-87%), p=0.0215 | ||
Source: Adapted from FDA Review
1 P-value was not statistically controlled for multiple comparisons. Abbreviations: ADAS-Cog 13, Alzheimer’s Disease Assessment Scale-Cognitive 13-task subscale; ADCS ADL MCI, Alzheimer's Disease
Cooperative Study - Activities of Daily Living Scale for use in Mild Cognitive Impairment; CDR-SB, Clinical Dementia Rating Scale Sum of Boxes; MMSE, Mini-Mental State Examination; NPI 10, Neuropsychiatric Inventory 10-item
Table 4 presents amyloid beta PET and CSF biomarker results from Trial 2.
Table 4. Biomarker Results of ADUHELM in Trial 2
| Biomarker Endpoint at Week 781 | ADUHELM High Dose |
Placebo |
|---|---|---|
| Amyloid beta PET composite SUVR | N=183 | N=204 |
| Mean baseline | 1.407 | 1.376 |
| Change from baseline | -0.235 | -0.003 |
| Difference from placebo | -0.232, p<0.0001 | |
| Amyloid beta PET centiloid | N=183 | N=204 |
| Mean baseline | 90.8 | 83.8 |
| Change from baseline (%) | -54.0 (-59%) | -0.5 |
| Difference from placebo | -53.5, p<0.0001 | |
| CSF p-tau (pg/mL) | N=18 | N=15 |
| Mean baseline | 121.81 | 94.53 |
| Change from baseline | -13.19 | -2.24 |
| Difference from placebo | -10.95, p=0.3019 | |
| CSF t-tau (pg/mL) | N=16 | N=14 |
| Mean baseline | 618.50 | 592.57 |
| Change from baseline | -102.51 | -33.26 |
| Difference from placebo | -69.25, p=0.3098 | |
Source: Adapted from FDA Review
1 P-values were not statistically controlled for multiple comparisons.
Abbreviations: CSF, cerebral spinal fluid; PET, positron emission tomography; p-tau, phosphorylated tau protein; SUVR, standard uptake value ratio; t-tau, total tau protein
No statistically significant differences were observed between the ADUHELM-treated and placebo-treated patients on the primary efficacy endpoint, the change from baseline in Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) score at 78 weeks in Trial 2.
Table 5 presents amyloid beta PET results from Trial 3.
Table 5. Biomarker Results of ADUHELM in Trial 3
| Biomarker Endpoint at Week 541 | ADUHELM 10 mg/kg |
Placebo |
|---|---|---|
| Amyloid beta PET composite SUVR | N=28 | N=42 |
| Mean baseline | 1.432 | 1.441 |
| Change from baseline | -0.263 | 0.014 |
| Difference from placebo | -0.277, p<0.0001 | |
| Amyloid beta PET centiloid | N=28 | N=42 |
| Mean baseline | 94.5 | 96.5 |
| Change from baseline (%) | -58.0 (-61%) | 3.1 |
| Difference from placebo | -61.1, p<0.0001 | |
Source: Adapted from FDA Review 1 P-values were not statistically controlled for multiple comparisons.
Abbreviations: CSF, cerebral spinal fluid; PET, positron emission tomography; p-tau, phosphorylated tau protein; SUVR, standard uptake value ratio; t-tau, total tau protein
Clinical assessments in Trial 3 were exploratory. Results for clinical assessments were directionally aligned with the findings from Trial 1, with less change from baseline in CDR-SB and Mini-Mental State Examination (MMSE) scores at 1 year in the ADUHELM 10 mg/kg fixed dose group than in patients on placebo (CDR-SB: -1.26, 95% CI [-2.356, -0.163]; MMSE: 1.9, 95% CI [0.06, 3.75]).
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ADUHELM worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how ADUHELM worked among races could not be determined.
- Age: ADUHELM worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Figure 1 shows the primary clinical efficacy endpoint results by sex and age subgroups for Trial 1. Race subgroups were not analyzed in the figure since most of the patients in the study were White. However, the number of Asian patients in placebo and high dose groups in the analysis are 47 and 40, respectively, and number White are 424 and 406, respectively. There were also 67 and 76, respectively, classified as ‘Other’ (which includes the 13% overall for which race was not reported, due to confidentiality reasons in some regions). In the Asian subgroup the placebo LS Mean change from baseline on CDR-SB at Week 78 was 1.47 and the high dose LS Mean at Week 78 was 0.39. In the White subgroup the placebo LS Mean at Week 78 was 1.80 and the high dose LS Mean at Week 78 was 1.40.
Figure 1. Week 78 CDR-SB Subgroup Analyses, Baseline Demographics, Trial 1
Source: Adapted from FDA Review
What are the possible side effects?
ADUHELM can cause serious side effects, including amyloid related imaging abnormalities (ARIA) and allergic reactions. ARIA is a common side effect that does not usually cause any symptoms but can be serious. It most commonly occurs as swelling in areas of the brain (ARIA-edema) that usually resolves with time. Some people may also have small spots of bleeding in or on the surface of the brain (ARIA-hemorrhage) with the swelling. Although most people with ARIA do not have symptoms, some people have symptoms such as headache, confusion, dizziness, vision changes, and nausea. Allergic reactions associated with ADUHELM could result in swelling of the face, eyes, lips, or tongue, which can lead to difficulty in swallowing or breathing.
The most common side effects of ADUHELM include ARIA, headache, and fall.
What are the possible side effects (results of trials used to assess safety)?
Table 6 below summarizes adverse reactions in patients treated with ADUHELM in Trials 1 and 2 at the recommended dose.
Table 6. Adverse Reactions Reported in at Least 2% of Patients Treated With ADUHELM 10 mg/kg and at Least 2% Higher than Placebo in Trials 1 and 2
| Adverse Reaction | ADUHELM 10 mg/kg N=1105 % |
Placebo N=1087 % |
|---|---|---|
| ARIA-edema | 35 | 3 |
| Headachea | 21 | 16 |
| ARIA-hemorrhage microhemorrhage | 19 | 7 |
| ARIA-hemorrhage superficial siderosis | 15 | 2 |
| Fall | 15 | 12 |
| Diarrheab | 9 | 7 |
| Confusion/Delirium/Altered mental status/Disorientationc | 8 | 4 |
Source: ADUHELM Prescribing Information
a Headache includes the adverse reaction related terms headache, head discomfort, migraine, migraine with aura, and occipital neuralgia.
b Diarrhea includes the adverse reaction related terms diarrhea and infectious diarrhea.
c Confusion/Delirium/Altered mental status/Disorientation includes the adverse reaction related terms confusional state, delirium, altered state of consciousness, disorientation, depressed level of consciousness, disturbance in attention, mental impairment, mental status changes, postoperative confusion, and somnolence.
Abbreviations: ARIA, amyloid related imaging abnormalities
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females, except for ARIA-Hemorrhage superficial siderosis, which occurred more frequently in males.
- Race: The number of patients of races other than White was small; therefore, differences in the occurrence of side effects could not be determined.
- Age: The occurrence of side effects was similar in different age groups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
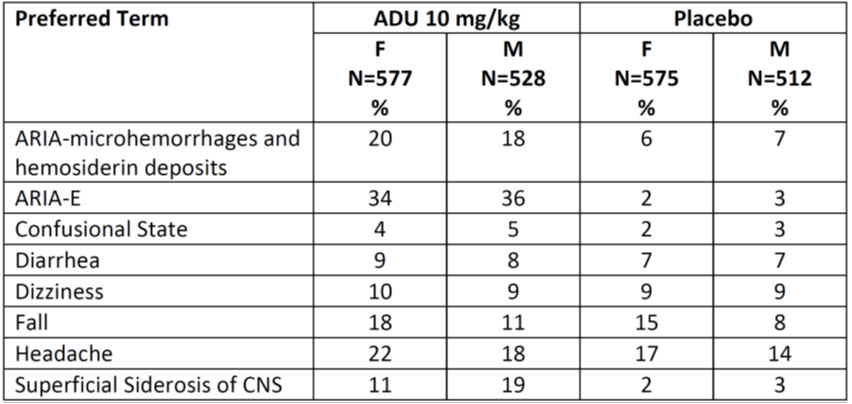
Table 7 and Table 8 below summarize the occurrence of adverse events by demographic subgroups in a pooled analysis of Trials 1 and 2.
Table 7. Incidence of Common Treatment Emergent Adverse Events in Pooled Trials 1 and 2 by Sex
Source: Adapted from FDA Review.
Abbreviations: ADU, ADUHELM; ARIA, amyloid related imaging abnormalities; CNS, central nervous system; E, edema; F, female; M, male
Table 8. Incidence of Common Treatment Emergent Adverse Events in Pooled Trials 1 and 2 by Age Group (Years)
Source: Adapted from FDA Review
Abbreviations: ADU, ADUHELM; ARIA, amyloid related imaging abnormalities; CNS, central nervous system; E, edema
DEMOGRAPHICS SNAPSHOT
Who participated in the trials?
Figure 2 summarizes how many male and female patients were enrolled in the clinical trials.
Figure 2. Demographics by Sex
Source: Adapted from FDA Review
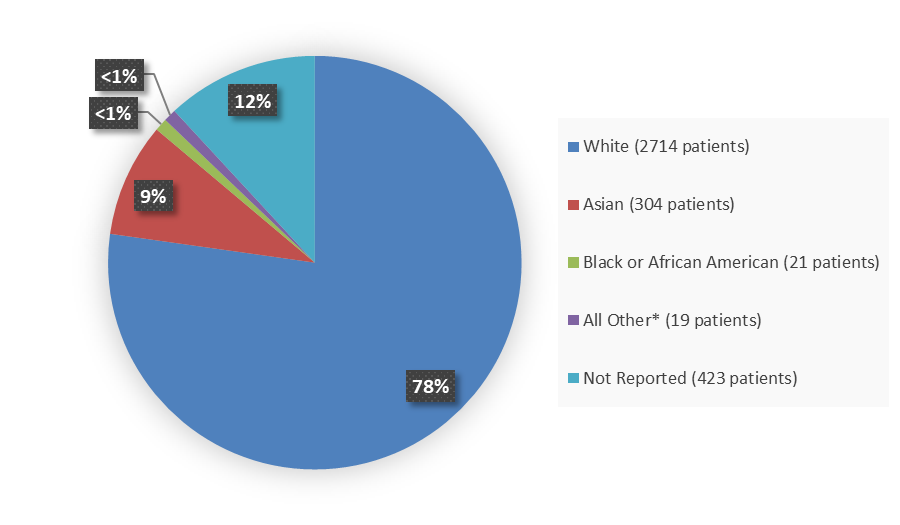
Figure 3 summarizes the percentage of patients by race enrolled in the clinical trials.
Figure 3. Demographics by Race
Source: Adapted from FDA Review
*Includes American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or Other
Figure 4 summarizes the percentage of patients by age enrolled in the clinical trials.
Figure 4. Demographics by Age
Source: Adapted from FDA Review
How were the trials designed?
ADUHELM was evaluated in 3 clinical trials that enrolled patients with Alzheimer’s disease.
Trials 1 and 2 enrolled patients with Alzheimer’s disease (patients with brain amyloid and mild cognitive impairment or mild dementia stage of disease). Patients were randomly assigned to receive either ADUHELM or placebo once every 4 weeks. Neither the patients nor the health care providers knew which treatment was being given. The benefit of ADUHELM compared to placebo was assessed after 78 weeks of treatment by comparing the change from baseline in the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB). In a subset of patients, the benefit was also evaluated by measuring the change in brain amyloid after 78 weeks of treatment and comparing it to placebo.
Trial 3 was a smaller study evaluating a range of doses in patients with Alzheimer’s disease (patients with brain amyloid and prodromal or mild dementia stage of disease). Patients were randomly assigned to receive either ADUHELM or placebo once every 4 weeks. Neither the patients nor the health care providers knew which treatment was being given. The benefit was evaluated by measuring the change in brain amyloid after 54 weeks of treatment and comparing it to placebo. Changes in CDR-SB were also assessed in the trial.
How were the trials designed?
The efficacy of ADUHELM was evaluated in two identically designed, double-blind, randomized, placebo-controlled studies (Trial 1 and Trial 2) in patients with Alzheimer’s disease (patients with confirmed presence of amyloid pathology and mild cognitive impairment or mild dementia stage of disease). Patients were randomized to receive ADUHLEM low dose, ADUHELM high dose, or placebo every 4 weeks for 18 months, followed by an optional, dose-blind, long-term extension period. Both studies included an initial titration period of up to 6 months to the maximum target dose. The primary efficacy endpoint was the change from baseline on the CDR-SB at Week 78. Secondary endpoints included the change from baseline in the Mini-Mental State Examination (MMSE) score at Week 78, the change from baseline in the Alzheimer’s Disease Assessment Scale-Cognitive 13-Task Subscale (ADAS-Cog 13) at Week 78, and the change from baseline in the Alzheimer’s Disease Cooperative Study - Activities of Daily Living Inventory for use in Mild Cognitive Impairment (ADCS-ADL-MCI) score at Week 78.
Trials 1 and 2 were terminated prior to their planned completion.
The effects of ADUHELM were also supported by a double-blind, randomized, placebo-controlled, dose-ranging study (Trial 3) in patients with Alzheimer’s disease (patients with confirmed presence of amyloid pathology and prodromal or mild dementia stage of disease). Patients were randomized to a range of ADUHELM doses for 12 months. The study was primarily designed to assess safety and tolerability, but also included pharmacodynamic endpoints (change in brain amyloid) and exploratory clinical assessments (CDR-SB and MMSE).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.