Drug Trials Snapshots: APHEXDA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the APHEXDA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
APHEXDA (motixafortide)
ah fex’ dah
BioLineRx, Ltd.
Approval date: September 8, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
APHEXDA is a prescription drug that in combination with filgrastim (a granulocyte-colony stimulating factor [G-CSF]) mobilizes hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma.
How is this drug used?
APHEXDA is an injection that is taken once after filgrastim has been administered daily for four days. A second dose of APHEXDA can be administered 10 to 14 hours prior to a third apheresis.
Who participated in the clinical trials?
The FDA approved APHEXDA based on evidence from a clinical trial of 122 patients with multiple myeloma due to undergo autologous transplantation. The trial was conducted at 21 sites in 5 countries including Italy, Hungary, Germany, Spain, and the United States.
There were 78 patients included in the trial from the United States, and 44 patients included from sites outside of the United States.
The same trial (GENESIS) was used to assess efficacy and safety.
How were the trials designed?
APHEXDA was evaluated in the GENESIS study, a double-blind, placebo-controlled study, in which 122 subjects with multiple myeloma were randomized 2:1 to receive APHEXDA 1.25 mg/kg with G-CSF (N=80) or placebo with G-CSF (N=42) for mobilization of hematopoietic stem cells for collection and apheresis. This one trial evaluated the benefit and side effects of APHEXDA in patients.
The benefit of APHEXDA was based on the proportion of patients who achieved a cell collection goal of ≥6×106 CD34+ cells/kg in up to two aphereses after administration of filgrastim and a single administration of APHEXDA or placebo.
How were the trials designed?
The safety and efficacy of APHEXDA were established in one trial of 122 patients with multiple myeloma due for mobilization of hematopoietic stem cells for collection and apheresis.
This was a randomized, double-blind, placebo-controlled trial in 122 patients (GENESIS, NCT03246529). Prior to receiving APHEXDA or placebo, patients received daily morning doses of filgrastim 10 to 15 µg/kg for four days. On the evening of Day 4, patients received APHEXDA or placebo. On Day 5, patients received a fifth morning dose of filgrastim within 1 hour prior to their first apheresis (12 hours ±2 hours from the APHEXDA or placebo administration).
The efficacy of APHEXDA was based upon the proportion of patients who achieved a cell collection goal of ≥6×106 CD34+ cells/kg in up to two aphereses after administration of filgrastim and a single administration of APHEXDA or placebo.
DEMOGRAPHICS SNAPSHOT:
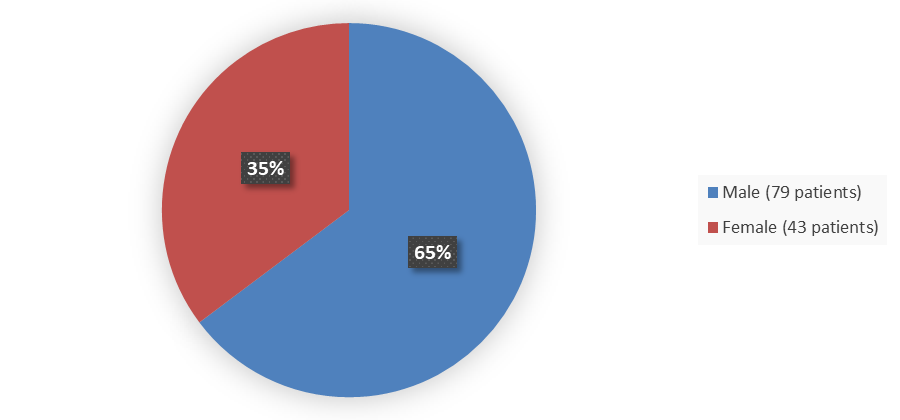
Figure 1 summarizes how many male and female patients were enrolled in the main clinical trial used to evaluate the efficacy of APHEXDA.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
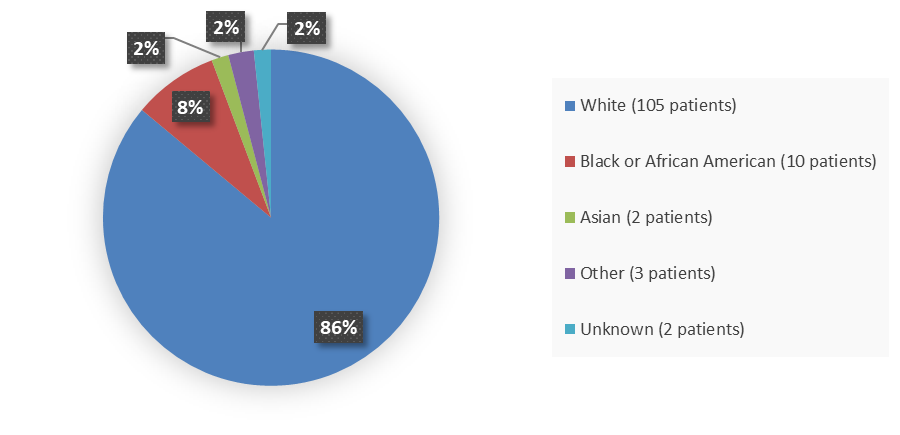
Figure 2 summarizes the percentage of patients by race enrolled in the main clinical trial used to evaluate the efficacy of APHEXDA.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
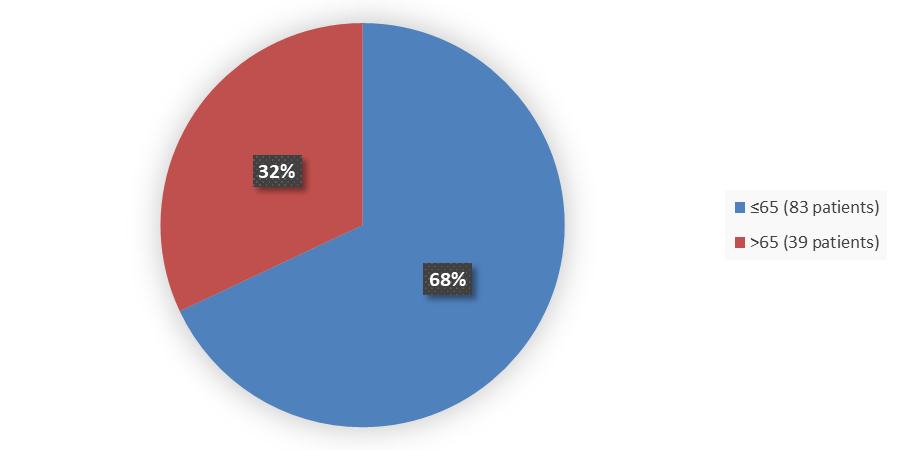
Figure 3 summarizes how many patients by age were in the main trial used to evaluate the side effects of APHEXDA.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
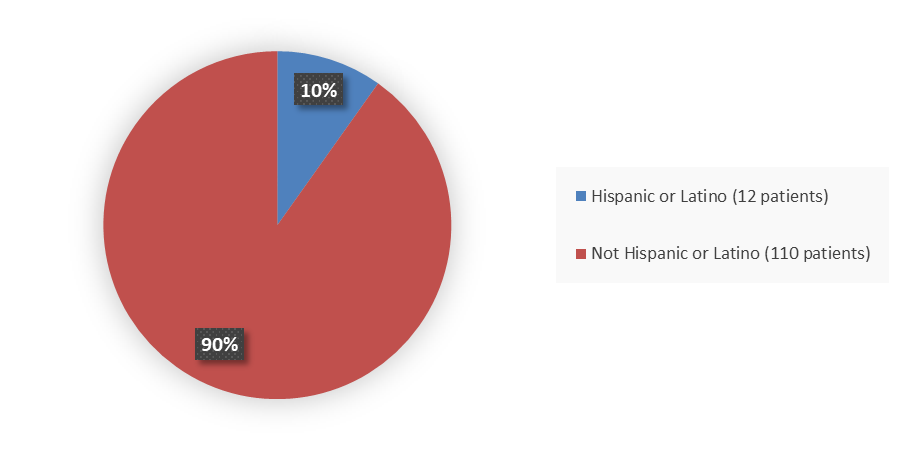
Figure 4 summarizes how many patients by ethnicity were in the combined trials used to evaluate the side effects of APHEXDA.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics, Safety Population, Trial GENESIS
| Characteristic |
APHEXDA + Filgrastim |
Placebo + Filgrastim |
|
Sex, n (%) |
|
|
|
Female |
25 (31.2) |
18 (42.9) |
|
Male |
55 (68.8) |
24 (57.1) |
|
Age, years |
|
|
|
Mean (SD) |
60.4 (9.4) |
59.2 (9.6) |
|
Median (min, max) |
63.5 (35, 75) |
62 (34, 74) |
|
Age group, years, n (%) |
|
|
|
≤65 |
53 (66.2) |
30 (71.4) |
|
>65 |
27 (33.8) |
12 (28.6) |
|
Age group ≥65, years, n (%) |
|
|
|
≥65 |
37 (46.2) |
17 (40.5) |
|
Age group ≥75, years, n (%) |
|
|
|
≥75 |
1 (1.2) |
0 |
|
Race, n (%) |
|
|
|
White |
65 (81.2) |
40 (95.2) |
|
Asian |
2 (2.5) |
0 |
|
Black or African American |
8 (10.0) |
2 (4.8) |
|
Other |
3 (3.8) |
0 |
|
Unknown |
2 (2.5) |
0 |
|
Ethnicity, n (%) |
|
|
|
Hispanic or Latino |
9 (11.2) |
3 (7.1) |
|
Not Hispanic or Latino |
71 (88.8) |
39 (92.9) |
|
Country of participation, n (%) |
|
|
|
United States |
52 (65.0) |
26 (61.9) |
|
Germany |
3 (3.8) |
0 |
|
Spain |
6 (7.5) |
2 (4.8) |
|
Hungary |
10 (12.5) |
6 (14.3) |
|
Italy |
9 (11.2) |
8 (19.0) |
|
Region of participation, n (%) |
|
|
|
Europe |
28 (35.0) |
16 (38.1) |
|
Non-Washington University (United States) |
28 (35.0) |
12 (28.6) |
|
Washington University (United States) |
24 (30.0) |
14 (33.3) |
|
Is in United States, n (%) |
|
|
|
United States |
52 (65.0) |
26 (61.9) |
|
Non-United States |
28 (35.0) |
16 (38.1) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
A significant number of patients with multiple myeloma preparing for hematopoietic transplant were able to mobilize a sufficient amount of cells in up to two apheresis sessions after a single administration of APHEXDA compared to placebo. This allows substantially more subjects to reach the apheresis goal even in one apheresis session in order to proceed to transplantation.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy of APHEXDA in combination with G-CSF was evaluated in the GENESIS study, a randomized, double-blind, placebo-controlled study in which 122 subjects with multiple myeloma were randomized 2:1 to receive APHEXDA 1.25 mg/kg with G-CSF (80 patients) or placebo with G-CSF (42 patients) for mobilization of hematopoietic stem cells for collection and apheresis.
Table 2. Proportion of Patients Who Achieved CD34+ Cell Collection Goal Following Single Administration of APHEXDA or Placebo (GENESIS)
|
Cell Collection Goal |
APHEXDA + Filgrastim N=80 |
Placebo + Filgrastim N=42 |
|
Proportion of patients with ≥6×106 CD34+ cells/kg in up to two aphereses |
67.5% |
9.5% |
|
Proportion of patients with ≥6×106 CD34+ cells/kg in one apheresis |
63.8% |
2.4% |
|
Proportion of patients with ≥2×106 CD34+ cells/kg in one apheresis |
87.5% |
38.1% |
Source: Adapted from APHEXDA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: APHEXDA worked similarly in males and females.
- Race: The majority of patients were White; therefore, differences in how well APHEXDA worked between races could not be determined because of the small number of patients of races other than White.
- Age: APHEXDA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes efficacy results based on difference in proportions between treatment arms by sex and age. Because of the small sample size, these exploratory analyses should be interpreted with caution.
Table 3. Difference in Proportion by Demographics
|
Subgroup |
n |
Proportional Difference |
95% CI |
|
Age, years |
|
|
|
|
≤65 |
83 |
0.56 |
0.39, 0.73 |
|
>65 |
39 |
0.48 |
0.19, 0.77 |
|
Sex |
|
|
|
|
Male |
79 |
0.56 |
0.36, 0.76 |
|
Female |
43 |
0.44 |
0.18, 0.70 |
Source: Clinical Trial Data
What are the possible side effects?
APHEXDA can cause injection site reactions, anaphylactic shock, and hypersensitivity reactions. Tumor cell mobilization in patients with leukemia, leukocytosis, potential for tumor cell mobilization, and embryo-fetal toxicity can also be caused by APHEXDA.
The most common side effects of APHEXDA are injection site reactions, pruritus, flushing, and back pain.
What are the possible side effects (results of trials used to assess safety)?
Table 4 summarizes the adverse reactions in the clinical trial occurring at a rate in ≥10% APHEXDA-treated patients and ≥2% more than placebo-treated patients.
Table 4. Adverse Reactions of Patients Receiving APHEXDA
|
Adverse Reaction |
APHEXDA + Filgrastim N=92 % |
Placebo + Filgrastim N=42 % |
|
Injection site reactionsa |
73 |
5 |
|
Injection site pain |
53 |
5 |
|
Injection site erythema |
27 |
0 |
|
Injection site pruritis |
24 |
0 |
|
Pruritis |
38 |
0 |
|
Flushingb |
33 |
0 |
|
Rashc |
16 |
5 |
|
Urticaria |
14 |
0 |
|
Erythema |
12 |
0 |
|
Back paind |
21 |
17 |
|
Paresthesiae |
19 |
17 |
|
Hypokalemia |
15 |
12 |
|
Nausea |
14 |
12 |
Source: Adapted from APHEXDA Prescribing Information
a Injection site reactions includes injection site bruising, injection site discomfort, injection site erythema, injection site induration, injection site mass, injection site nodule, injection site pain, injection site pruritus, injection site rash, injection site swelling, injection site urticaria, injection site cellulitis, and injection related reaction.
b Flushing includes hot flush.
c Rash includes rash erythematous, rash maculo-papular, rash popular, and rash pruritic.
dBack pain includes spinal pain and sacral pain.
e Paresthesia includes paresthesia oral, hypoesthesia, hypoesthesia oral, and burning sensation.
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- *Race: The number of patients other than White and Black were very small, therefore the differences in the occurrence of side effects could not be determined.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
*In Trials 1 and 2, the number of patients of races other than White and Black was small; therefore, differences in how OMLONTI worked among races could not be determined. Trial 3 was exclusively conducted in Asia therefore analysis by race is not conducted for this study.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5. Overview of Adverse Events by Demographic Subgroup
|
Characteristic |
APHEXDA + Filgrastim |
Placebo + Filgrastim |
Risk Difference |
|
Sex |
|
|
|
|
Female |
24/25 (96.0) |
15/18 (83.3) |
12.7 (‑6.2, 31.5) |
|
Male |
53/55 (96.4) |
20/24 (83.3) |
13.0 (‑2.7, 28.7) |
|
Age group, years |
|
|
|
|
≤65 |
51/53 (96.2) |
24/30 (80.0) |
16.2 (1.0, 31.4) |
|
>65 |
26/27 (96.3) |
11/12 (91.7) |
4.6 (‑12.6, 21.8) |
|
Age group ≥65, years |
|
|
|
|
≥65 |
35/37 (94.6) |
15/17 (88.2) |
6.4 (‑10.6, 23.3) |
|
Age group ≥75, years |
|
|
|
|
≥75 |
1/1 (100) |
0/0 (NA) |
NA |
|
Race |
|
|
|
|
White |
62/65 (95.4) |
34/40 (85.0) |
10.4 (‑1.8, 22.6) |
|
Asian |
2/2 (100) |
0/0 (NA) |
NA |
|
Black or African American |
8/8 (100) |
1/2 (50.0) |
50.0 (‑19.3, 119.3) |
|
Other |
3/3 (100) |
0/0 (NA) |
NA |
|
Unknown |
2/2 (100) |
0/0 (NA) |
NA |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
9/9 (100) |
3/3 (100) |
0 (0, 0) |
|
Not Hispanic or Latino |
68/71 (95.8) |
32/39 (82.1) |
13.7 (0.8, 26.6) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; N, number of patients in treatment arm; n, number of patients meeting criteria; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.