Drug Trials Snapshots: BREXAFEMME
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the BREXAFEMME Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
BREXAFEMME (ibrexafungerp)
(Brex ä fem)
Scynexis Inc.
Approval date: June 1, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BREXAFEMME is a drug used for the treatment of vaginal yeast infection (vulvovaginal candidiasis) in adult or adolescent females who have already begun having monthly menstrual cycles.
How is this drug used?
BREXAFEMME is a tablet. Two tablets are taken by mouth approximately 12 hours apart for one day.
BREXAFEMME tablets may be administered approximately 12 hours apart (e.g., in the morning and in the evening) for one day, for a total daily dosage of 600 mg (four 150-mg tablets). BREXAFEMME may be taken with or without food. Take BREXAFEMME exactly as your healthcare provider tells you to take it.
Who participated in the clinical trials?
The FDA approved BREXAFEMME based on evidence from clinical trials of 820 patients with vulvovaginal candidiasis. The trials were conducted at 70 sites in 2 countries: the United States and Bulgaria. The same trials were used to assess efficacy and safety. The efficacy population included all patients with a baseline vaginal culture positive for Candida species of yeast who took at least 1 dose of study medication, while the safety population included all patients who took at least 1 dose of study medication. The number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
Who participated in the trials?
Table 1. Demographic Characteristics (Trial 1 and Trial 2 Safety Population)
| Subgroup | BREXAFEMME N=545 n (%) |
Placebo N=275 n (%) |
Total N=820 n (%) |
|---|---|---|---|
| Sex | |||
| Female | 545 (100.0) | 275 (100.0) | 820 (100.0) |
| Age group (years) | |||
| <18 | 0 (0.0) | 1 (0.4) | 1 (0.1) |
| 18 to 64 | 540 (99.1) | 269 (97.8) | 809 (98.7) |
| >65 | 5 (0.9) | 5 (1.8) | 10 (1.2) |
| Race | |||
| White | 378 (69.4) | 194 (70.5) | 572 (69.8) |
| Black or African American | 152 (27.9) | 78 (28.4) | 230 (28.0) |
| Asian | 4 (0.7) | 0 (0.0) | 4 (0.5) |
| American Indian or Alaska Native | 3 (0.6) | 1 (0.4) | 4 (0.5) |
| Other | 8 (1.5) | 2 (0.7) | 10 (1.2) |
Source: Adapted from FDA Review
What are the benefits of this drug?
More patients treated with BREXAFEMME had complete resolution of signs and symptoms of vaginal yeast infection (e.g., redness, itching, or burning) than patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The determination of efficacy of BREXAFEMME was based on the proportion of patients who had a complete clinical response at the Test of Cure (TOC) visit occurring between Day 8 to 14 in Trials 1 and 2. Additional endpoints included a negative culture for Candida species at the TOC visit (also known as the mycological response), and clinical outcome at the follow-up visit occurring between Day 21 to 29.
Table 2. Clinical and Mycological Response (Trial 1 and Trial 2 mITT/Efficacy Population)1
| Parameter | Trial 1 | Trial 2 | ||
|---|---|---|---|---|
| BREXAFEMME N=190 n (%) |
Placebo N=100 n (%) |
BREXAFEMME N=189 n (%) |
Placebo N=89 n (%) |
|
| Complete clinical response at TOC2 | 95 (50.0) | 28 (28.0) | 120 (63.5) | 40 (44.9) |
| Difference (95% CI) | 22.0 (10.2, 32.8) | 18.6 (6.0, 30.6) | ||
| P-value | 0.001 | 0.009 | ||
| Negative culture at TOC | 94 (49.5) | 19 (19.0) | 111 (58.7) | 26 (29.2) |
| Difference (95% CI) | 30.5 (19.4, 40.3) | 29.5 (17.2, 40.6) | ||
| P-value | <0.001 | <0.001 | ||
| Complete clinical response at follow-up3 | 113 (59.5) | 44 (44.0) | 137 (72.5) | 44 (49.4) |
| Difference (95% CI) | 15.5 (3.4, 27.1) | 23.1 (10.8, 35.0) | ||
| P-value | 0.007 | 0.006 | ||
Source: BREXAFEMME prescribing information Section 14
1 The mITT/efficacy population included randomized subjects with a baseline culture positive for Candida species who took at least 1 dose of study medication
2 Absence of signs and symptoms (VSS Score of 0) without need for additional antifungal therapy or topical drug therapy for the treatment of vulvovaginal symptoms at test of cure (TOC) visit.
3 Absence of signs and symptoms (VSS Score of 0) without need for further antifungal treatment or topical drug therapy for the treatment of vulvovaginal symptoms prior to follow-up visit.
Abbreviations: mITT, modified intent-to-treat; VSS, vulvovaginal signs and symptoms
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All trial participants were female; therefore, sex differences cannot be determined.
- Race: Approximately 69% of trial participants were White and 28% were Black or African American patients and BREXAFEMME appears to work similarly in both groups.
- Age: Very few females older than 65 years of age were enrolled. BREXAFEMME appears to work similarly in patients below and above 35 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The primary efficacy endpoint (clinical cure at the TOC visit, Day 8 to 14) by various subgroups is summarized in Table 3 and Table 4 for the modified intent-to-treat (mITT)/efficacy population. Interpretation of these results must be made with caution given lack of type 1 error control for multiple analyses and the limited sample size in some of the subgroup categories. The difference between treatment groups was generally comparable for most subgroups and supportive of the overall population.
Table 3. Clinical Cure at TOC for Various Subgroups in Trial 1 (mITT/Efficacy Population)1
| Subgroup | BREXAFEMME n/N (%) |
Placebo n/N (%) |
Difference (95% CI)2 |
|---|---|---|---|
| Race | |||
| White | 49/103 (47.6) | 13/55 (23.6) | 23.9 (8.2, 37.7) |
| Black | 40/74 (51.1) | 14/43 (32.6) | 21.5 (2.7, 38.4) |
| Asian | 4/4 (100) | - | - |
| Other | 2/9 (22.2) | 1/2 (50.0) | -27.8 (-77.5, 30.1) |
| Age (years) | |||
| <18 | - | 0/1 (0.0) | - |
| 18 to 35 | 55/108 (50.9) | 16/56 (28.6) | 22.3 (6.5, 36.5) |
| ≥36 | 40/82 (48.8) | 12/43 (27.9) | 20.9 (2.7, 36.9) |
Source: Adapted from FDA review Table 8-7; Reviewer conducted analyses using ADSL and ADEFF datasets
1 The mITT/efficacy population included randomized subjects with a baseline culture positive for Candida species who took at least 1 dose of study medication
2 Difference (BREXAFEMME - placebo) and 95% confidence interval is based on Miettinen and Nurminen’s method
Abbreviations: mITT, modified intent-to-treat; TOC, test of cure
Table 4. Clinical Cure at TOC for Various Subgroups in Trial 2 (mITT/Efficacy Population)1
| Subgroup | BREXAFEMME n/N (%) |
Placebo n/N (%) |
Difference (95% CI)2 |
|---|---|---|---|
| Race | |||
| White | 103/154 (66.9) | 33/70 (47.1) | 19.7 (5.7, 33.1) |
| Black | 16/34 (47.1) | 7/19 (36.8) | 10.2 (-17.3, 35.5) |
| Other | 1/1 | - | - |
| Age (years) | |||
| 18 to 35 | 72/118 (61.0) | 22/56 (39.3) | 21.7 (5.9,36.5) |
| ≥36 | 48/71 (67.6) | 18/33 (54.6) | 13.1 (-6.9, 32.5) |
Source: Adapted from FDA review Table 8-18; Reviewer conducted analyses using ADSL and ADEFF dataset
1 The mITT/efficacy population included randomized subjects with a baseline culture positive for Candida species who took at least 1 dose of study medication
2 Difference (BREXAFEMME - placebo) and 95% confidence interval is based on Miettinen and Nurminen’s method
Abbreviations: mITT, modified intent-to-treat; TOC, test of cure
What are the possible side effects?
The most common side effects are diarrhea, nausea, abdominal pain, dizziness, and vomiting.
BREXAFEMME should not be taken during pregnancy based on findings of birth defects in rabbit studies. Pregnancy status should be verified before BREXAFEMME is prescribed. Patients should take appropriate steps to prevent pregnancy during treatment and for four days after the last dose.
What are the possible side effects?
Table 5 summarizes the most common adverse reactions observed in the trials.
Table 5. Adverse Reactions With Rates ≥2% in BREXAFEMME-Treated Patients (Trial 1 and Trial 2), Safety Population1
| Adverse Reaction | BREXAFEMME N=545 n (%) |
Placebo N=275 n (%) |
|---|---|---|
| Diarrhea | 91 (16.7%) | 9 (3.3%) |
| Nausea | 65 (11.9%) | 11 (4.0%) |
| Abdominal pain2 | 62 (11.4%) | 14 (5.1%) |
| Dizziness3 | 18 (3.3%) | 7 (2.5%) |
| Vomiting | 11 (2.0%) | 2 (0.7%) |
Source: BREXAFEMME prescribing information Section 6.1
1 The safety population included randomized subjects who took at least 1 dose of study medication
2 Includes abdominal pain, abdominal pain upper, abdominal pain lower, and abdominal discomfort
3 Includes dizziness and postural dizziness
Were there any differences in side effects among sex, race and age?
- Sex: All trial participants were female; therefore, sex differences cannot be determined.
- Race: Approximately 69% of trial participants were White and 28% were Black or African American patients and the occurrence of side effects appears similar between both groups.
- Age: Very few females older than 65 years of age were enrolled, therefore, differences in side effects by age below and above 65 years could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6 summarizes the overall incidence of the adverse events reported in the combined trials by race. Since all the patients were female and few were older than 65 years, analyses by sex and age were not included. The incidence of adverse events was generally comparable between racial groups.
Table 6. Incidence of Treatment Emergent Adverse Events by Race – Trial 1 and Trial 2, Safety Population
| Parameter | BREXAFEMME | Placebo | ||
|---|---|---|---|---|
| N | n (%) | N | n (%) | |
| Race | ||||
| White | 378 | 160 (42.3) | 194 | 71 (36.6) |
| Black or African American | 152 | 82 (53.9) | 78 | 35 (44.9) |
| Asian | 4 | 2 (50.0) | 0 | 0 |
| Other | 11 | 5 (45.5) | 3 | 1 (33.3) |
Source: Adapted from BREXAFEMME FDA review, Section 8.2.7, Table 8-39
DEMOGRAPHICS SNAPSHOT
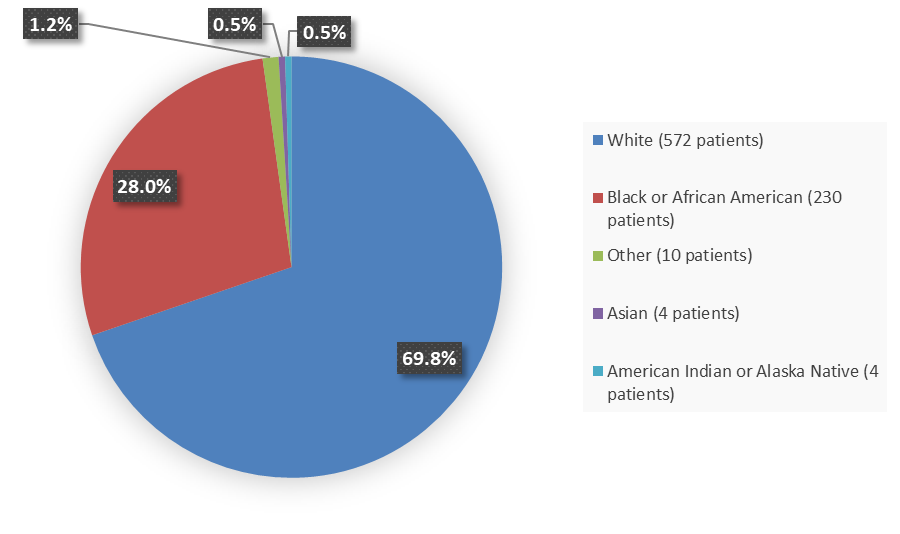
Figure 1 summarizes the percentage of patients by race enrolled in the combined clinical trials used to evaluate the efficacy of BREXAFEMME.
Figure 1. Baseline Demographics by Race (Trial 1 and Trial 2 Efficacy Population)
Source: Adapted from FDA Review
Figure 2 summarizes how many patients by race were in the combined trials used to evaluate the side effects of BREXAFEMME.
Figure 2. Baseline Demographics by Race (Trial 1 and Trial 2 Safety Population)
Source: Adapted from FDA Review
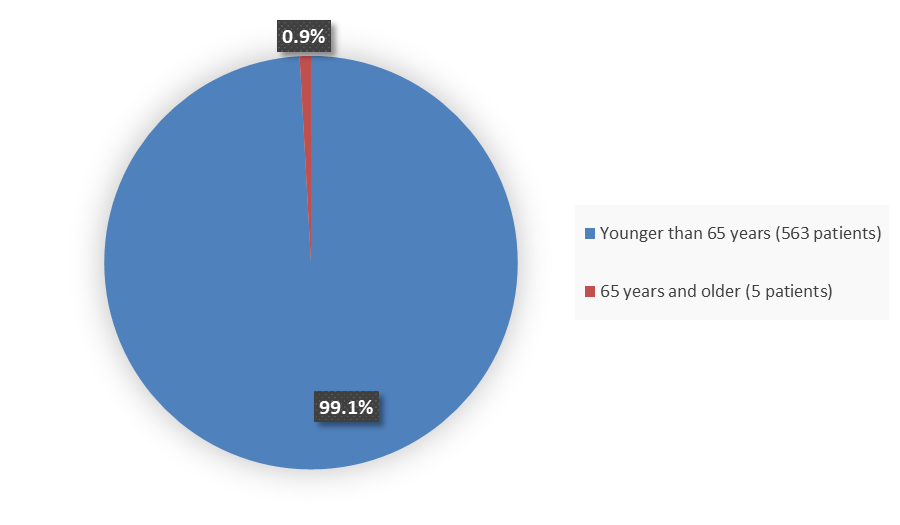
Figure 3 summarizes how many patients by age were in the combined trials used to evaluate the efficacy of BREXAFEMME.
Figure 3. Baseline Demographics by Age (Trial 1 and Trial 2 Efficacy Population)
Source: Adapted from FDA Review
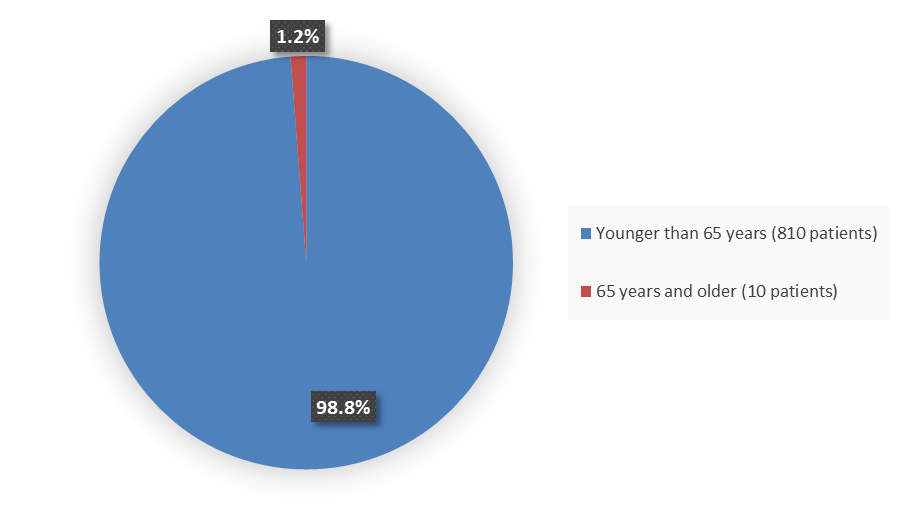
Figure 4 summarizes how many patients by age were in the combined trials used to evaluate the side effects of BREXAFEMME.
Figure 4. Baseline Demographics by Age (Trial 1 and Trial 2 Safety Population)
Source: Adapted from FDA Review
How were the trials designed?
There were 2 multicenter, randomized, placebo-controlled trials designed to evaluate the safety and efficacy of BREXAFEMME in adult or adolescent females who have already begun having monthly menstrual cycles and who had vulvovaginal candidiasis. The efficacy of BREXAFEMME was assessed at 8 to 14 days post-treatment in both trials and compared to placebo. The primary efficacy endpoint was a complete clinical response, defined as the complete resolution of signs and symptoms of vulvovaginal candidiasis.
How were the trials designed?
Two randomized placebo-controlled clinical trials (Trial 1, NCT03734991 and Trial 2, NCT03987620) with a similar design were conducted to evaluate the safety and efficacy of a single day of BREXAFEMME 600 mg (two 150-mg tablets per dose, administered 12 hours apart) for the treatment of vulvovaginal candidiasis (VVC). Non-pregnant, post-menarchal females with a diagnosis of VVC were eligible. A diagnosis of VVC was defined as: (a) minimum composite vulvovaginal signs and symptoms (VSS) score of ≥4 with at least two signs or symptoms having a score of 2 (moderate) or greater; (b) positive microscopic examination with 10% KOH in a vaginal sample revealing yeast forms (hyphae/pseudohyphae) or budding yeasts; and (c) normal vaginal pH (≤4.5). The total composite VSS score was based on the vulvovaginal signs (erythema, edema, excoriation) and vulvovaginal symptoms (itching, burning, or irritation) where each was scored as 0 = absent, 1 = mild, 2 = moderate, or 3 = severe.
Study visits included the test of cure (TOC, Day 8 to 14) visit and a follow-up (Day 21 to 29) visit. The modified intent-to-treat (mITT) population was used to assess the efficacy endpoints and included randomized subjects with a baseline culture positive for Candida species who took at least 1 dose of study medication.
Efficacy was assessed by clinical outcome at the TOC visit. A complete clinical response was defined as the complete resolution of signs and symptoms (VSS score of 0). Additional endpoints included a negative culture for Candida species at the TOC visit, and clinical outcome at the follow-up visit.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.