Drug Trials Snapshots: ELREXFIO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ELREXFIO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ELREXFIO (elranatamab-bcmm)
el-reks-fe-o
Pfizer, Inc.
Approval date: August 14, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ELREXFIO is a drug used to treat a form of blood cancer called multiple myeloma. It is to be used in patients whose cancer came back after, or did not respond to, at least four previous treatment regimens, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
How is this drug used?
ELREXFIO is given by a healthcare provider as an injection under the skin (subcutaneous injection). ELREXFIO is given at lower “step-up” doses during the first week. After the first full “treatment” dose, ELREXFIO is given once every week. Later, it may be given every two weeks.
The healthcare provider will decide how many treatment cycles will be given.
Who participated in the clinical trials?
The FDA approved ELREXFIO based on evidence from a clinical trial (NCT04649359) of 187 patients with relapsed or refractory multiple myeloma. The same trial was used to assess efficacy and safety. Safety findings were analyzed from 183 patients who received ELREXFIO at the recommended dosing regimen. The efficacy of ELREXFIO was based on 97 patients whose cancer came back after, or did not respond to, at least four prior treatment regimens, which included a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. The trial was conducted at 53 study centers in 10 countries.
How were the trials designed?
ELREXFIO was evaluated in a clinical trial of 187 adult patients with multiple myeloma whose disease came back after, or did not respond to, previous treatments. All patients in the trial received ELREXFIO until the disease progressed or the side effects became too toxic.
How were the trials designed?
The efficacy and safety of ELREXFIO was evaluated in patients with relapsed or refractory multiple myeloma in an open-label, single arm, multi-center study (NCT04649359). The study included patients with multiple myeloma whose disease came back after or did not respond to treatment that included at least one proteasome inhibitor, one immunomodulatory agent, and one anti-CD38 monoclonal antibody.
Eligible patients received subcutaneous administration of ELREXFIO at step-up doses of 12 mg on Day 1 and 32 mg on Day 4 of treatment, followed by the first treatment dose of ELREXFIO (76 mg) on Day 8 of treatment. Thereafter, patients received 76 mg once weekly. After 24 weeks, in patients who achieved an International Myeloma Working Group (IMWG) response category of partial response or better with responses persisting for at least two months, the dose interval was changed from every week to every two weeks.
The primary endpoint of the trial was the objective response rate (ORR), defined as the proportion of patients who achieved a partial response or better according to IMWG response criteria.
DEMOGRAPHICS SNAPSHOT
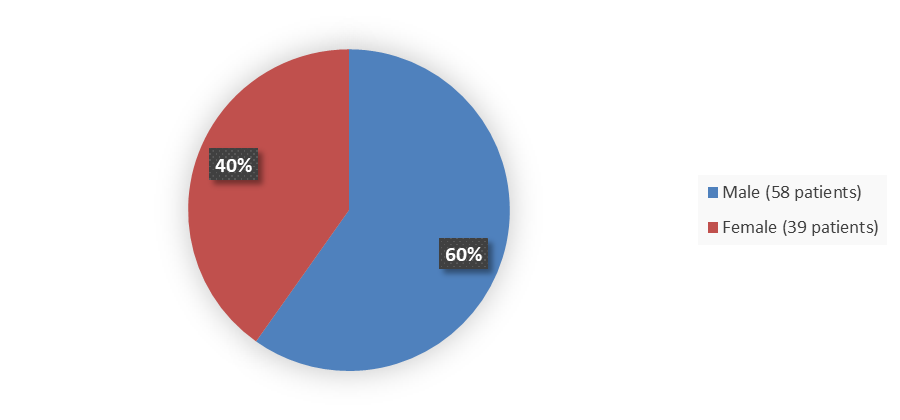
Figure 1 summarizes the percentage of males and females in the clinical trial used to evaluate the efficacy of ELREXFIO.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
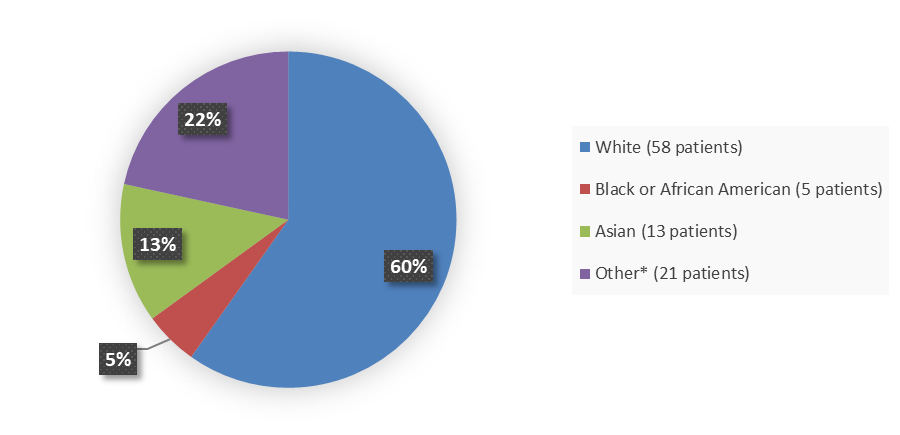
Figure 2 summarizes how many patients by race were enrolled in the clinical trial used to evaluate the efficacy of ELREXFIO.
Figure 2. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
* Other includes unknown and not reported race patients
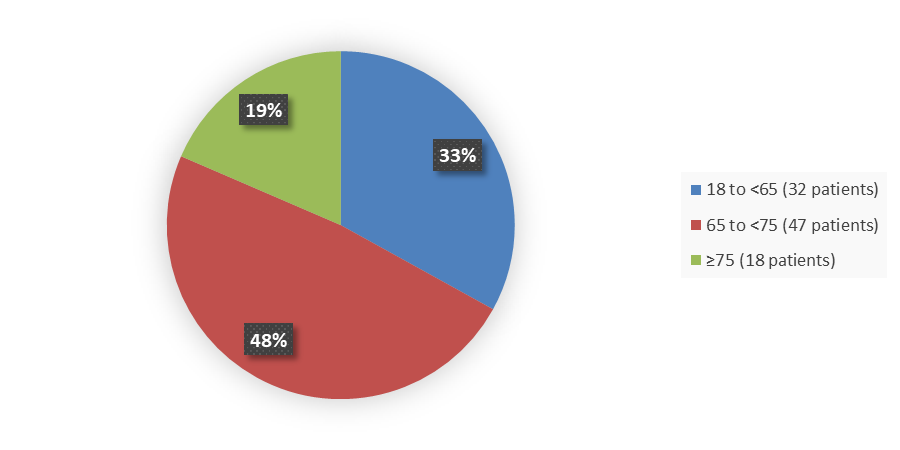
Figure 3 summarizes how many patients by age were enrolled in the clinical trial used to evaluate the efficacy of ELREXFIO.
Figure 3. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
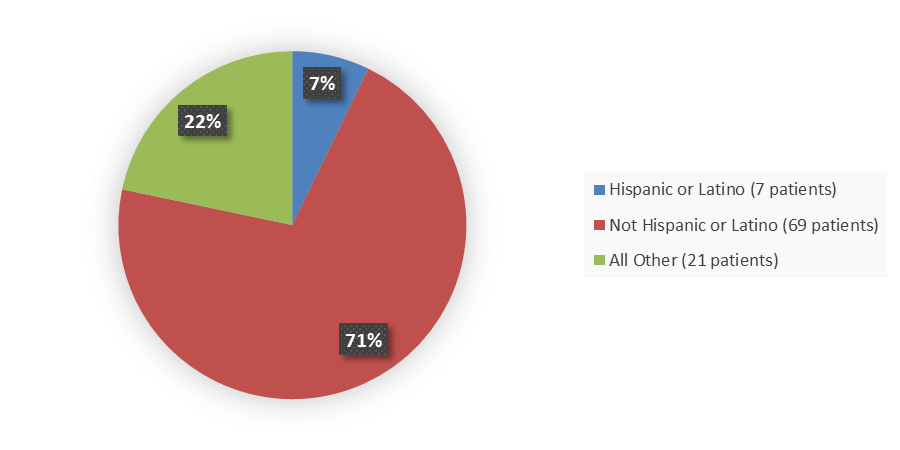
Figure 4 summarizes how many patients by ethnicity were enrolled in the clinical trial used to evaluate the efficacy of ELREXFIO.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
* Other includes not reported and missing ethnicity patients
Who participated in the trials?
Table 1. Demographics, Efficacy Population
|
Demographic Characteristic |
ELREXFIO N=97 |
|
Sex, n (%) |
|
|
Male |
58 (59.8) |
|
Female |
39 (40.2) |
|
Race, n (%) |
|
|
White |
58 (59.8) |
|
Black or African American |
5 (5.2) |
|
Asian |
13 (13.4) |
|
Unknown |
2 (2.1) |
|
Not reported |
19 (19.6) |
|
Age, years |
|
|
Mean (SD) |
67.3 (9.16) |
|
Median (min, max) |
69 (46, 89) |
|
Age group, years, n (%) |
|
|
18 to <65 |
32 (33.0) |
|
65 to <75 |
47 (48.5) |
|
≥75 |
18 (18.6) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
7 (7.2) |
|
Not Hispanic or Latino |
69 (71.1) |
|
Not reported |
20 (20.6) |
|
Missing |
1 (1.0) |
|
Geographic region, n (%) |
|
|
North America |
44 (45.4) |
|
Europe |
37 (38.1) |
|
Asia |
9 (9.3) |
|
Other |
7 (7.2) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
Table 2. Demographics, Safety Population
|
Demographic Characteristic |
ELREXFIO N=183 |
|
Sex, n (%) |
|
|
Male |
95 (51.9) |
|
Female |
88 (48.1) |
|
Race, n (%) |
|
|
White |
112 (61.2) |
|
Black of African American |
11 (6.0) |
|
Asian |
17 (9.3) |
|
Unknown |
3 (1.6) |
|
Not reported |
40 (21.9) |
|
Age, years |
|
|
Mean (SD) |
66.5 (9.36) |
|
Median (min, max) |
68.0 (36, 88) |
|
Age group, years, n (%) |
|
|
18 to <65 |
70 (38.3) |
|
65 to <75 |
78 (42.6) |
|
≥75 |
35 (19.1) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
18 (9.8) |
|
Not Hispanic or Latino |
115 (62.8) |
|
Not reported |
48 (26.2) |
|
Missing |
2 (1.1) |
|
Geographic region, n (%) |
|
|
North America |
91 (49.7) |
|
Europe |
71 (38.8) |
|
Asia |
12 (6.6) |
|
Other |
9 (4.9) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
In the trial, 56 of 97 patients treated with ELREXFIO experienced an improvement in their disease. For 90% of patients who responded, that improvement lasted at least six months. For 80% of patients who responded, that improvement lasted for at least nine months.
ELREXFIO was approved under the FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of ELREXFIO was evaluated by measuring ORR, as assessed by an independent review committee based on the IMWG Response Criteria for Multiple Myeloma.
Table 3 summarizes the efficacy results for the 97 patients that comprised the primary efficacy population in the clinical trial.
Table 3. Efficacy Results
|
Endpoint |
ELREXFIO N=97 |
|
Objective response rate1, n (%) |
56 (57.7) |
|
ORR 95% CI |
47.3, 67.7 |
|
Complete response or better2, n (%) |
25 (25.8) |
|
Very good partial response, n (%) |
25 (25.8) |
|
Partial response, n (%) |
6 (6.2) |
|
Duration of response, months |
|
|
Median (95% CI) |
NR (12.0, NE) |
Source: Adapted from FDA Review
1 Objective response rate = sCR + CR + VGPR + PR
2 Complete response or better = sCR + CR
Abbreviations: CI, confidence interval; CR, complete response; NE, not estimable; NR, not reached; ORR, objective response rate; PR, partial response; sCR, stringent complete response; VGPR, very good partial response
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: ELREXFIO worked similarly in males and females.
- Race: Most patients who received ELREXFIO in this study were White, and the numbers in other race groups were too small to assess whether ELREXFIO worked better or worse in those groups.
- Age: ELREXFIO worked similarly in patients younger than 65 and 65 to 74 years of age. The number of patients 75 years of age and above was too small to assess whether ELREXFIO worked better or worse in those patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 4 summarizes the subgroup analysis of ORR. Overall, the results across subgroups were consistent with the primary efficacy analysis. The interpretation of the results across race and for patients 75 years of age and older is limited due to the small sample size of the study.
Table 4. Subgroup Analysis of ORR, Efficacy Population
|
Subgroup |
ELREXFIO, N=97 |
|
|
ORR n/N (%) |
95% CI |
|
|
Sex |
|
|
|
Male |
30/58 (51.7) |
38.2, 65.0 |
|
Female |
26/39 (66.7) |
49.8, 80.9 |
|
Race |
|
|
|
White |
35/58 (60.3) |
46.6, 73.0 |
|
Black or African American |
2/5 (40.0) |
5.3, 85.3) |
|
Asian |
8/13 (61.5) |
31.6, 86.1 |
|
Age, years |
|
|
|
<65 |
15/32 (46.9) |
29.1, 65.3 |
|
65 to <75 |
31/47 (66.0) |
50.7, 79.1 |
|
≥75 |
10/18 (55.6) |
30.8, 78.5 |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; ORR, objective response rate
What are the possible side effects?
ELREXFIO may cause side effects that are serious, life-threatening, or lead to death, including cytokine release syndrome (CRS) and neurologic problems, including a neurologic problem called immune effector cell-associated neurotoxicity syndrome (ICANS). Because of the CRS and neurologic problems, including ICANS, ELREXFIO is available only through a drug safety program called ELREXFIO Risk Evaluation Mitigation Strategy.
Other serious side effects include infections, decreased white blood cell counts, liver problems, and harm to an unborn baby.
The most common side effects include: CRS; tiredness; injection site reaction, such as redness, itching, pain, bruising, rash, swelling, or tenderness; diarrhea; upper respiratory tract infection; muscle and bone pain; pneumonia; decreased appetite; rash; cough; nausea; and fever. These are not all the possible side effects of ELREXFIO.
What are the possible side effects (results of trials used to assess safety)?
Table 5 summarizes adverse reactions in the 183 patients who received ELREXFIO at the recommended dosing regimen in the clinical trial.
Table 5 summarizes adverse reactions in the 183 patients who received ELREXFIO at the recommended dosing regimen in the clinical trial.
| Adverse Reactions |
ELREXFIO, N=183 |
|
|
All Grades % |
Grade 3 or 4 % |
|
|
Immune system disorders |
|
|
|
Cytokine release syndrome |
58 |
0.5# |
|
Hypogammaglobulinemia* |
13 |
2.2# |
|
General disorders and site administration conditions |
|
|
|
Fatigue* |
43 |
6# |
|
Injection site reaction* |
37 |
0 |
|
Pyrexia |
21 |
2.7# |
|
Edema* |
18 |
1.1# |
|
Gastrointestinal disorders |
|
|
|
Diarrhea |
36 |
1.1# |
|
Nausea |
22 |
0 |
|
Constipation |
15 |
0 |
|
Vomiting |
14 |
0 |
|
Infections |
|
|
|
Upper respiratory tract infection* |
34 |
4.9 |
|
Pneumoniaa |
32 |
19 |
|
Sepsisb |
15 |
11 |
|
Urinary tract infection* |
12 |
4.4# |
|
Musculoskeletal and connective tissue disorders |
|
|
|
Musculoskeletal pain* |
34 |
2.7# |
|
Metabolism and nutrition disorders |
|
|
|
Decreased appetite |
26 |
1.1# |
|
Skin and subcutaneous tissue disorders |
|
|
|
Rashc |
25 |
0 |
|
Dry skin |
13 |
0 |
|
Skin exfoliation* |
10 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
|
|
|
Cough* |
24 |
0 |
|
Dyspnea* |
15 |
3.3# |
|
Nervous system disorders |
|
|
|
Headache |
18 |
0.5 |
|
Encephalopathyd |
15 |
2.7 |
|
Sensory neuropathye |
13 |
0.5# |
|
Motor dysfunctionf |
13 |
2.2# |
|
Cardiac disorders |
|
|
|
Cardiac arrhythmia* |
16 |
2.2 |
|
Vascular disorders |
|
|
|
Hemorrhage* |
13 |
1.6 |
|
Psychiatric disorders |
|
|
|
Insomnia |
13 |
0 |
|
Injury, poisoning and procedural complications |
|
|
|
Fall |
10 |
0.5# |
Source: ELREXFIO Prescribing Information
* Includes other related terms
a Pneumonia included COVID-19 pneumonia, lower respiratory tract infection, lower respiratory tract infection viral, pneumocystis jirovecii pneumonia, pneumonia, pneumonia adenoviral, pneumonia bacterial, pneumonia cytomegaloviral, pneumonia fungal, pneumonia influenzal, pneumonia pseudomonal, and pneumonia viral.
b Sepsis includes bacteremia, device related bacteremia, device related sepsis, escherichia bacteremia, escherichia sepsis, klebsiella sepsis, pseudomonal sepsis, sepsis, septic shock, staphylococcal bacteremia, staphylococcal sepsis, streptococcal sepsis, and urosepsis.
c Rash included erythema, palmar-plantar erythrodysesthesia syndrome, rash, rash erythematous, rash macular, rash maculo-papular, rash pustular, symmetrical drug-related intertriginous, and flexural exanthema.
d Encephalopathy included agitation, altered state of consciousness, cognitive disorder, confusional state, delirium, depressed level of consciousness, disorientation, hallucination, lethargy, memory impairment, mental status changes, metabolic encephalopathy, somnolence, and toxic encephalopathy.
e Sensory neuropathy included burning sensation, dysesthesia, hypoesthesia, neuropathy peripheral, paresthesia, parosmia, peripheral sensorimotor neuropathy, peripheral sensory neuropathy, polyneuropathy, and sensory loss.
f Motor dysfunction included ataxia, balance disorder, gait disturbance, motor dysfunction, muscle contracture, muscle spasms, muscular weakness, peripheral motor neuropathy, peroneal nerve palsy, and tremor.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: Most patients who received ELREXFIO in this study were White, and the numbers in other race groups were too small to assess whether there were differences in the occurrence of side effects among race groups.
- Age: The occurrence of side effects was similar across age groups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6 and Table 7 summarize adverse reactions by subgroups in the 183 patients who received ELREXFIO at the recommended dosing regimen in the clinical trial.
Table 6. Summary of Adverse Reaction by Sex
| Adverse Reaction Category |
Male N=95 n (%) |
Female N=88 n (%) |
|
Any AR |
95 (100) |
88 (100) |
|
CRS |
51 (54) |
55 (63) |
|
Any AR grade 3 or 4 |
63 (66) |
67 (76) |
|
Serious AR |
67 (71) |
58 (66) |
Source: Adapted from FDA Review
Abbreviations: AR, adverse reaction; CRS, cytokine release syndrome
Table 7. Summary of Adverse Reactions by Age
|
Adverse Reaction Category |
<65 Years N=70 n (%) |
65 to <75 Years N=78 n (%) |
≥75 Years N=35 n (%) |
|
Any AR |
70 (100) |
78 (100) |
35 (100) |
|
CRS |
43 (61) |
47 (60) |
16 (46) |
|
Any AR grade 3 or 4 |
49 (70) |
58 (74) |
23 (66) |
|
Serious AR |
47 (67) |
54 (69) |
24 (69) |
Source: Adapted from FDA Review
Abbreviations: AR, adverse reaction; CRS, cytokine release syndrome
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION