Drug Trials Snapshots: EMFLAZA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to EMFLAZA Prescribing Information for complete information.
EMFLAZA (deflazacort)
(em fla’ zah)
Marathon Pharmaceuticals, LLC
Approval date: February 9, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
EMFLAZA is a corticosteroid drug for the treatment of Duchenne muscular dystrophy (DMD) in patients 5 years of age and older.
Duchenne muscular dystrophy is a rare disease that affects primarily boys. It is caused by a low level or lack of one muscle protein called dystrophin. Lack of dystrophin causes progressive muscle weakness and premature death.
How is this drug used?
EMFLAZA is taken by mouth once a day in the form of a pill or liquid at the recommended dose.
What are the benefits of this drug?
EMFLAZA improved muscle strength of DMD patients.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of EMFLAZA was evaluated in Trial 1 by assessing the change in the average muscle strength scores from Baseline to Week 12 in a total of 18 muscle groups for each patient. Patients were asked to perform different muscle strength assessments in various positions, with each test being graded using a modified Medical Research Council (MRC) 11-point scale. A score of 0 was consistent with no movement of the muscle, while a score of 10 indicated normal strength.
Table 2. Analysis of Change from Baseline at Week 12 in Average Muscle Strength Score (Trial 1)
| Treatment | N | Change from Baseline LS Mean (95% CI) | EMFLAZA-Placebo | P-value |
|---|---|---|---|---|
| EMFLAZA 0.9 mg/kg/day | 51 | 0.15 (0.01, 0.28) | 0.25 | 0.017 |
| Placebo | 50 | -0.10 (-0.23, 0.03) | - | - |
LS=least squares
CI=confidence interval
Adapted from FDA Clinical review
In Trial 2, efficacy results suggested less decline of strength for EMFLAZA with numerical differences at 6 and 12 months compared to placebo.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DMD occurs almost entirely in males; therefore, differences in response between sexes could not be determined.
- Race: The majority of the patients were white, with few patients of other races; therefore, differences in side effects among races could not be determined.
- Age: EMFLAZA worked similarly in patients who were 5-9 years old and in patients who were 9-15 years old.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subpopulation analyses were not conducted for sex and race given that all the patients in the trials were boys and the majority was white. Efficacy analysis by age group (below and above the age of 9) is presented below in Table 3.
Table 3. Subgroup Efficacy Analysis by Age
| EMFLAZA 0.9 mg/kg/d | Placebo | |
|---|---|---|
| Baseline Age 9> | ||
| n | 21* | 30 |
| Active-Placebo | 0.24 | - |
| 95% CI | (-0.001, 0.474) | - |
| Baseline Age ≥9 years | ||
| n | 28* | 20 |
| Active-Placebo | 0.31 | - |
| 95% CI | (0.095, 0.518) | - |
*one patient missing post-baseline score
CI=Confidence Interval
Adapted from FDA Statistical review
What are the possible side effects?
EMFLAZA, like other corticosteroid drugs may cause some serious side effects such as hormonal suppression, infections, heart and kidney problems, stomach or gut rupture, changes in mood and behavior, weakening of the bones, and some eye disorders.
The most common side effects are Cushingoid appearance (hormonal condition causing facial puffiness), weight gain, increased appetite, upper respiratory tract infection, cough, frequent urination, excessive hairiness, and increased fat around the waist.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes common adverse reactions that occurred in patients treated with EMFLAZA from Trial 1. Presented is safety population defined as all patients who received at least one dose of either EMFLAZA at recommended dose or placebo.
Table 4. Adverse Reactions that Occurred in ≥ 5% of EMFLAZA-Treated Patients and Occurred More Frequently than in Placebo Patients with DMD (Trial 1-safety population)
| Adverse Reaction | EMFLAZA 0.9 mg/kg/d (N=51) % at 12 weeks | Placebo (N=50) % at 12 weeks1 |
|---|---|---|
| Cushingoid appearance | 33 | 12 |
| Weight increased | 20 | 6 |
| Increased appetite | 14 | 2 |
| Upper respiratory tract infection | 12 | 10 |
| Cough | 12 | 6 |
| Pollakiuria | 12 | 2 |
| Nasopharyngitis | 10 | 6 |
| Hirsutism | 10 | 2 |
| Central obesity | 10 | 4 |
| Erythema | 8 | 6 |
| Irritability | 8 | 4 |
| Rhinorrhea | 8 | 0 |
| Abdominal discomfort | 6 | 2 |
1 At 12 weeks placebo patients were re-randomized to receive either EMFLAZA or an active comparator.
EMFLAZA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: DMD occurs almost entirely in males; therefore, differences in side effects between sexes could not be determined.
- Race: The majority of the patients were white, with few patients of other races; therefore, differences in side effects among races could not be determined.
- Age: The risk of side effects was similar in patients who were 5-9 years old and in patients who were 9-15 years old.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Subpopulation analyses were not conducted for sex and race given that all the patients in the trials were boys and the majority was white. Adverse reaction analyses by age group (below and above the age of 9) are presented below in Tables 5 and 6, respectively.
Table 5. Subgroup Analysis of Adverse Reactions in Age Group 5-9 years (Trial 1-safety population)
| Adverse Reaction | EMFLAZA 0.9 mg/kg/d (N=22) % at 12 weeks | Placebo (N=30) % at 12 weeks1 |
|---|---|---|
| Cushingoid appearance | 18 | 7 |
| Weight increased | 5 | 0 |
| Increased appetite | 9 | 3 |
| Upper respiratory tract infection | 14 | 10 |
| Cough | 18 | 10 |
| Pollakiuria | 5 | 0 |
| Nasopharyngitis | 18 | 3 |
| Hirsutism | 0 | 3 |
| Central obesity | 0 | 0 |
| Erythema | 5 | 7 |
| Irritability | 0 | 7 |
| Rhinorrhea | 14 | 0 |
| Abdominal discomfort | 5 | 3 |
Clinical trial data
Table 6. Subgroup Analysis of Adverse Reactions in Age Group 9-15 years (Trial 1-safety population)
| Adverse Reaction | EMFLAZA 0.9 mg/kg/d (N=29) % at 12 weeks | Placebo (N=20) % at 12 weeks1 |
|---|---|---|
| Cushingoid appearance | 45 | 20 |
| Weight increased | 31 | 15 |
| Increased appetite | 17 | 0 |
| Upper respiratory tract infection | 10 | 10 |
| Cough | 7 | 0 |
| Pollakiuria | 17 | 5 |
| Nasopharyngitis | 3 | 10 |
| Hirsutism | 17 | 0 |
| Central obesity | 17 | 10 |
| Erythema | 10 | 5 |
| Irritability | 14 | 0 |
| Rhinorrhea | 3 | 0 |
| Abdominal discomfort | 7 | 0 |
1 At 12 weeks placebo patients were re-randomized to receive either EMFLAZA or an active comparator.
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
There were two clinical trials that enrolled patients with Duchenne muscular dystrophy.
The FDA approved EMFLAZA based primarily on Trial 1 which enrolled 196 patients. One hundred one (101) patients received either EMFLAZA at the recommended dose or placebo. These 101 patients represent the main group of patients used for the assessment of EMFLAZA benefits and side effects and will be presented in the Figures 1, 2 and 3 below.
The rest of the patients from Trial 1 (95 patients) received either higher than recommended dose of EMFLAZA or an active comparator. These patients are presented in Table 7 under MORE INFO.
Trial 1 was conducted in the United States and Canada.
The FDA also considered data from 29 patients who participated in Trial 2 and received EMFLAZA or placebo. The dosing regimen for EMFLAZA was different from the recommended one. These patients are presented in Table 7 under MORE INFO. Trial 2 was conducted in Italy.
The figure below summarizes how many patients, by sex were in the clinical trial used to evaluate the benefit of the drug. This population is called the efficacy population.
Figure 1. Baseline Demographics by Sex (Trial 1-efficacy population)
Clinical trial data
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trial used to evaluate the benefit of the drug (efficacy population).
Figure 2. Baseline Demographics by Race (Trial 1-efficacy population)
Clinical trial data
Table 1. Baseline Demographics by Race (Trial 1-efficacy population)
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 95 | 94 |
| Other | 6 | 6 |
Clinical trial data
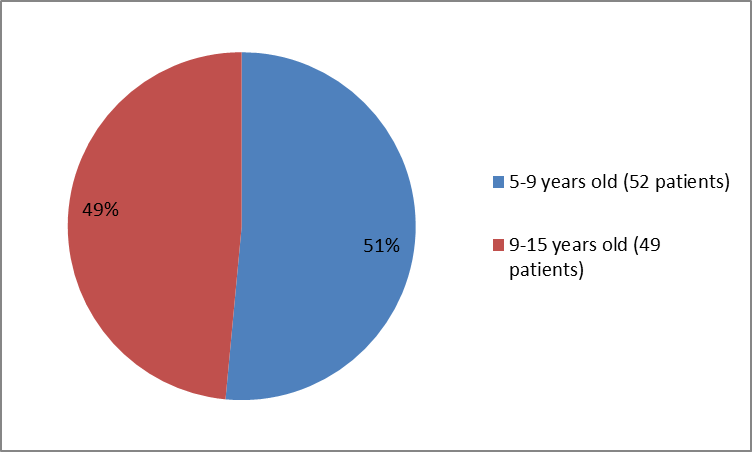
Figure 3 summarizes the percentage of patients by age group in Trial 1 who were used to evaluate the benefit of the drug (efficacy population).
Figure 3. Baseline Demographics by Age (Trial 1-efficacy population)
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 7. Baseline Demographics of Patients in the Clinical Trials
| Demographic Parameters | Trial 1 N=196 | Trial 2 N=29c n (%) | ||
|---|---|---|---|---|
| N=101a n (%) | N=95b n (%) | |||
| Sex | ||||
| Male | 101 (100) | 95 (100) | 29 (100) | |
| Age | ||||
| Median (years) | 9 | 8 | 8 | |
| Min, max (years) | 5, 15 | 5,15 | 6,10 | |
| Age Group | ||||
| 9=""> | 52 (51) | n/a | n/a | |
| ≥9 years | 49 (49) | n/a | n/a | |
| Race | ||||
| White | 95 (94) | 90 (95) | N/A* | |
| Asian | 0 (0) | 1 (1) | N/A* | |
| Black or African American | 0 (0) | 0 (0) | N/A* | |
| Other | 6 (6) | 4 (4) | N/A* | |
| Region | ||||
| Unites States/Canada | 101(100) | 95 (100) | 0 (0) | |
| Italy | 0 (0) | 0 (0) | 29 (100) | |
a EMFLAZA 0.9 mg/kg/day (recommended dosing) and placebo arm combined
b EMFLAZA (not recommended dosing) and active comparator arm combined
c EMFLAZA (not recommended dosing) and placebo arm combined
* Data not collected
Clinical trial data
How were the trials designed?
There were two trials that evaluated EMFLAZA.
In Trial 1, patients were randomly assigned to receive EMFLAZA (lower and higher dose), an active comparator, or placebo once a day for 12 weeks. The benefit was evaluated by the change in average muscle strength score from baseline to Week 12. After Week 12, patients who received placebo were switched to receive either EMFLAZA or the active comparator; all patients continued treatment for an additional 40 weeks. Neither the patients nor the health care providers knew which treatment was being given.
In Trial 2, patients received a higher dose of EMFLAZA every other day or placebo for 2 years. Neither the patients nor the health care providers knew which treatment was being given until the trial was completed. The benefit was evaluated by the change in average muscle strength score from baseline to 2 years or loss of ambulation, whichever occurred first.
How were the trials designed?
The safety and efficacy of EMFLAZA were evaluated in two clinical trials. Enrolled patients had to have an onset of Duchenne muscular dystrophy symptoms before the age of 5.
Trial 1 was a 52-week, randomized, placebo controlled, parallel-group trial. Patients were randomized to therapy with EMFLAZA (0.9 or 1.2 mg/kg/day), an active comparator, or placebo. The primary clinical efficacy endpoint was the change in average muscle strength score from baseline to Week 12 using a modified Medical Research Council (MRC) 11-point scale. A score of 0 was consistent with no movement of the muscle, while a score of 10 indicated normal strength.
After 12 weeks, placebo patients were re-randomized to receive either EMFLAZA or the active comparator; all patients continued treatment for an additional 40 weeks.
Trial 2 was a randomized, double-blind, placebo- controlled, 104-week trial. Patients were randomized to either high dose deflazacort every other day or placebo. The primary measure of efficacy was the average muscle strength change graded on a 0-5 scale from baseline to 2 years or loss of ambulation, whichever occurred first.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.