Drug Trials Snapshots: ENJAYMO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ENJAYMO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ENJAYMO (sutimlimab-jome)
(en-jaye’-moe)
Bioverativ Therapeutics, Inc.
Original Approval date: February 4, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ENJAYMO is a prescription drug that decreases the need for red blood cell (RBC) transfusion due to hemolysis (red blood cell destruction) in adults with cold agglutinin disease (CAD).
CAD is a rare blood disorder characterized by red blood cell destruction, which leads to anemia (a decrease in the number of RBCs) and cold-induced circulatory symptoms, such as pain and discoloration of fingers or toes. Many patients with CAD need RBC transfusions to manage their disease.
How is this drug used?
ENJAYMO is an infusion placed into a vein that is taken weekly for the first two weeks, followed by every two weeks thereafter.
Who participated in the clinical trials?
The FDA approved ENJAYMO based on evidence from one clinical trial of 24 patients with CAD. The trial was conducted at 16 sites in 8 of countries in Australia, Germany, France, Italy, Japan, Norway, United Kingdom, and the United States.
How were the trials designed?
ENJAYMO was evaluated in one clinical trial of 24 patients with CAD. This trial evaluated the benefit and side effects of ENJAYMO in patients with CAD. In this trial, patients had at least one blood transfusion within the past six months. Patients received ENJAYMO for up to six months and could choose to continue therapy in a second part of the trial. ENYAJMO was administered into the vein on Day 0, Day 7, and every 14 days through Week 25.
The benefit of ENJAYMO was evaluated based on response, which was defined by an increase in hemoglobin, no red blood cell transfusions after the first 5 weeks of treatment, and no other therapies for CAD as defined in the study.
How were the trials designed?
The safety and efficacy of ENJAYMO were established in one trial of 24 patients with CAD.
This was an open-label, single-arm, 6-month trial in 24 patients (CARDINAL, NCT03347396). Following the completion of the 6-month treatment period, patients continued to receive ENJAYMO in a long-term safety and durability of response extension phase for an additional 24 months. Patients with a confirmed diagnosis of CAD based on chronic hemolysis, polyspecific direct antiglobulin test (DAT), monospecific DAT specific for C3d, cold agglutinin titer ≥64 at 4°C, and IgG DAT ≤1+ and a recent blood transfusion in the 6 months prior to enrollment were administered 6.5 g or 7.5 g ENJAYMO (based on body weight) intravenously over approximately 60 minutes on Day 0, Day 7, and every 14 days thereafter through Week 25.
Efficacy was based on the proportion of patients who met the following criteria: an increase from baseline in Hgb level ≥2 g/dL or a Hgb level ≥12 g/dL at the treatment assessment time point (mean value from Weeks 23, 25, and 26), no blood transfusion from Week 5 through Week 26, and no treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26.
DEMOGRAPHICS SNAPSHOT
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of ENJAYMO.
Figure 1. Baseline Demographics by Sex (Safety Population)
Source: Clinical Trial Data
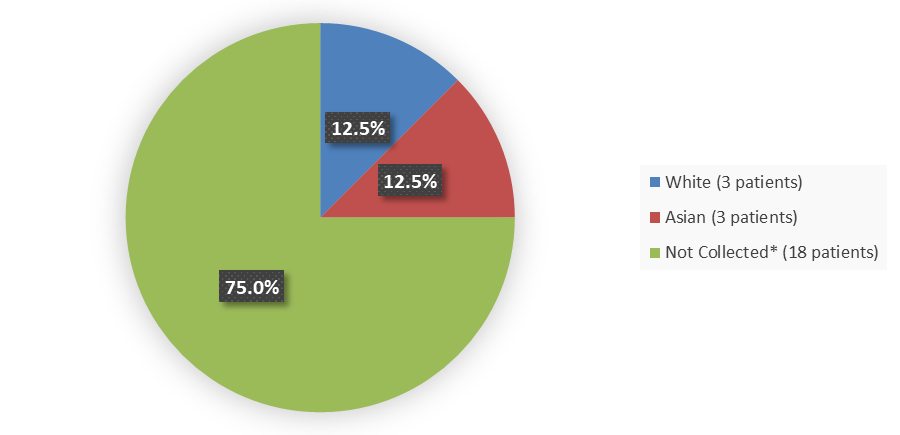
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of ENJAYMO.
Figure 2. Baseline Demographics by Race (Safety Population)
Source: Clinical Trial Data
* Data on race and/or ethnicity were not collected because of local regulations.
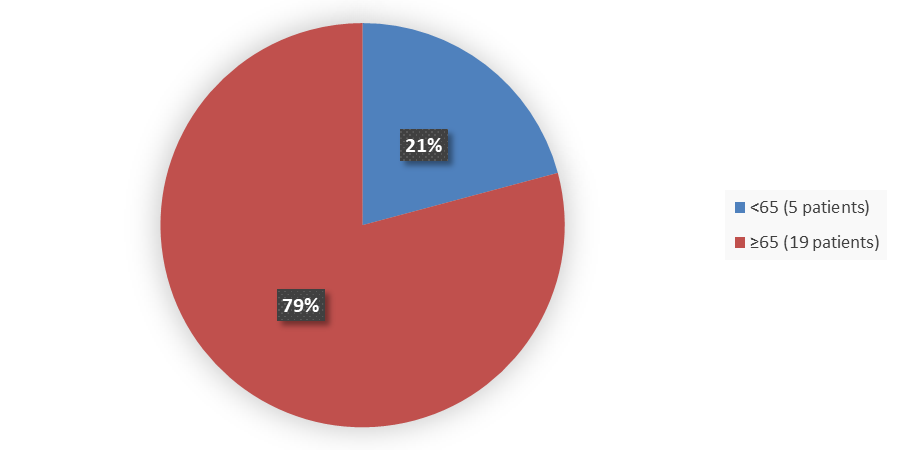
Figure 3 below summarizes how many patients by age were in the trial used to evaluate the side effects of ENJAYMO.
Figure 3. Baseline Demographics by Age (Safety Population)
Source: Clinical Trial Data
Who participated in the trials?
Table 5 summarizes demographics of the safety population in the clinical trial.
Table 5. Baseline Demographics of Patients in the Clinical Trial – Safety Population
|
|
ENJAYMO 6.5 g |
ENJAYMO 7.5 g |
Total |
|---|---|---|---|
|
Age, years |
|||
|
Mean (SD) |
71.8 (9.05) |
70.1 (6.01) |
71.3 (8.18) |
|
Median |
72.0 |
70.0 |
71.5 |
|
Min, max |
55, 85 |
63, 77 |
55, 85 |
|
Age group, years, n (%) |
|||
|
<65 |
3 (17.6) |
2 (28.6) |
5 (20.8) |
|
≥65 |
14 (82.4) |
5 (71.4) |
19 (79.2) |
|
Sex, n (%) |
|||
|
Female |
11 (64.7) |
4 (57.1) |
15 (62.5) |
|
Male |
6 (35.3) |
3 (42.9) |
9 (37.5) |
|
Race, n (%) |
|||
|
Asian |
3 (17.6) |
0 |
3 (12.5) |
|
White |
2 (11.8) |
1 (14.3) |
3 (12.5) |
|
Not collected1 |
12 (70.6) |
6 (85.7) |
18 (75.0) |
|
Ethnicity, n (%) |
|||
|
Hispanic or Latino |
0 |
0 |
0 |
|
Not Hispanic or Latino |
5 (29.4) |
1 (14.3) |
6 (25.0) |
|
Not collected1 |
12 (70.6) |
6 (85.7) |
18 (75.0) |
|
Region, n (%) |
|||
|
Europe |
12 (70.6) |
5 (71.4) |
17 (70.8) |
|
North America |
2 (11.8) |
1 (14.3) |
3 (12.5) |
|
Asia |
3 (17.6) |
0 |
3 (12.5) |
|
Other |
0 |
1 (14.3) |
1 (4.2) |
Source: Clinical Trial Data
1 Data on race and/or ethnicity were not collected because of local regulations.
Abbreviations: SD, standard deviation
What are the benefits of this drug?
After 6 months of treatment, 54% of patients responded to ENJAYMO. Response to treatment was defined based on changes on hemoglobin (Hgb) levels and whether a patient needed blood transfusion. Hgb is a protein in red blood cells that carries oxygen to tissues in the body.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 summarizes the efficacy results based on the response definition. Efficacy was based on the proportion of patients who met the following criteria: an increase from baseline in Hgb level ≥2 g/dL or a Hgb level ≥12 g/dL at the treatment assessment time point (mean value from Weeks 23, 25, and 26), no blood transfusion from Week 5 through Week 26, and no treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26.
Table 1. Efficacy Results in Patients With CAD (Full Analysis Set)
|
|
ENJAYMO |
|---|---|
|
Response rate |
13 (54.2) |
|
95% CI |
32.8, 74.4 |
|
Components of the primary endpoint |
|
|
Subjects with either Hgb ≥12 g/dL or increased ≥2 g/dL from baseline at treatment assessment timepoint |
15 (62.5) |
|
Subjects with Hgb ≥12 g/dL at treatment assessment timepoint |
9 (37.5) |
|
Subjects with Hgb increased ≥2 g/dL from baseline at treatment assessment timepoint |
15 (62.5) |
|
Subjects free of transfusions during Week 5 to Week 26 (EOT) |
17 (70.8) |
|
Subjects receiving no protocol-prohibited CAD medications1 during Week 5 to Week 26 (EOT) |
22 (91.7) |
Source: ENJAYMO Prescribing Information
1 Prohibited therapies include rituximab alone or in combination with cytotoxic agents.
Abbreviations: CAD, cold agglutinin disease; CI, confidence interval; EOT, end of treatment; Hgb, hemoglobin
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ENJAYMO worked better in female patients than male patients.
- Race: Race was not collected in most patients therefore differences in how ENJAYMO worked among races was not determined.
- Age: Differences in how well ENJAYMO worked among age groups could not be determined because of the small number of patients ≤65 years old.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 summarizes efficacy results based on response by sex and age. Because of the small sample size, these exploratory analyses should be interpreted with caution.
Table 2. Response Rate by Demographics
|
Subgroup |
Responder |
95% CI |
|---|---|---|
|
Age, years |
||
|
≤65 |
3/5 (60) |
14.7, 94.7 |
|
>65 |
10/19 (52.6) |
28.9, 75.6 |
|
Sex |
||
|
Male |
2/9 (22.2) |
2.8, 60.0 |
|
Female |
11/15 (73.3) |
44.9, 92.2 |
Source: Clinical Trial Data
Abbreviations: CI, confidence interval
What are the possible side effects?
ENJAYMO can cause serious infections, infusion-related reactions, and may increase the risk of developing autoimmune diseases. If ENJAYMO treatment is interrupted red blood cell breakdown may reoccur.
The most common side effects of ENJAYMO are respiratory tract infection, viral infection, diarrhea, dyspepsia (indigestion), cough, arthralgia (joint stiffness), arthritis, and swelling in the lower legs and hands.
What are the possible side effects (results of trials used to assess safety)?
Table 3 summarizes the adverse reactions in the clinical trial occurring at a rate ≥5% of patients treated with ENJAYMO.
Table 3. Adverse Reactions (≥5%) of Patients Receiving ENJAYMO
|
Adverse Reaction |
ENJAYMO |
|---|---|
|
Infections |
|
|
Respiratory tract infection*a |
6 (25) |
|
Viral infection*b |
3 (13) |
|
Urinary tract infection*c |
2 (8) |
|
Bacterial infection*d |
2 (8) |
|
Vascular disorders |
|
|
Cyanosis |
2 (8) |
|
Systemic hypertensione |
2 (8) |
|
Gastrointestinal disorders |
|
|
Diarrhea |
3 (13) |
|
Dyspepsiaf |
3 (13) |
|
Gastroenteritis |
2 (8) |
|
Abdominal pain |
2 (8) |
|
Respiratory, thoracic and mediastinal disorders |
|
|
Coughg |
3 (13) |
|
Musculoskeletal and connective tissue disorders |
|
|
Arthralgia, arthritis*h |
3 (13) |
|
General disorders and administration site conditions |
|
|
Peripheral edema |
3 (13) |
|
Fatigue*i |
2 (8) |
|
Infusion reaction |
2 (8) |
|
Nervous system disorders |
|
|
Headache |
2 (8) |
Source: ENJAYMO Prescribing Information
*Events may be counted in more than one grouped term, e.g., viral upper respiratory tract infection is counted in viral infection and respiratory tract infection.
a Includes nasopharyngitis, respiratory tract infection, respiratory tract infection viral, upper respiratory tract infection, and viral upper respiratory tract infection
b Includes oral herpes, respiratory tract infection viral, viral infection, and viral upper respiratory tract infection
c Includes cystitis bacterial and urinary tract infection
d Includes cystitis bacterial, streptococcal sepsis, and wound infection staphylococcal
e Includes hypertension and blood pressure increased
f Includes dyspepsia and abdominal pain upper
g Includes cough and productive cough
h Includes arthralgia and osteoarthritis
i Includes fatigue and mental fatigue
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: Differences in the occurrence of side effects between males and females could not be determined because of the small number of patients.
- Race: Race was not collected in most patients therefore differences in side effects among races could not be determined.
- Age: Differences in occurrence of side effects among age groups could not be determined because of the small number of patients ≤65 years old.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.