Drug Trials Snapshots: INMAZEB
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the INMAZEB Package Insert for complete information.
INMAZEB (atoltivimab, maftivimab, and odesivimab-ebgn)
ihn-ma-zehb
Regeneron
Approval date: October 14, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
INMAZEB is a drug used to treat pediatric and adult patients who have an infection caused by Zaire ebolavirus.
Zaire ebolavirus infection (sometimes called Ebola or Ebola virus disease) is a rare, contagious, rapidly progressive and often deadly disease.
How is this drug used?
INMAZEB is an injection. It is given one time by a healthcare provider directly into the vein (intravenous infusion). The amount of the drug to be administered is based on the patient’s weight.
What are the benefits of this drug?
INMAZEB lowered the risk of dying from the infection. Out of 154 patients treated with INMAZEB, 52 patients (34%) died within 28-days in comparison to 78 out the 153 patients (51%) who were treated with another experimental drug.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results for a pre-specified interim analysis of 28-day mortality. The table includes only the patients randomized and eligible to receive either INMAZEB or the control during the same time period of the trial. Treatment assignments were randomized within separate subgroups determined by the baseline laboratory test for Zaire ebolavirus infection corresponding to either a high viral load (CtNP ≤ 22.0) or a low viral load (CtNP ˃22.0).
Table 1. Mortality Rates in Trial 1
| Efficacy Endpoints | INMAZEBa (N=154) |
Controla (N=153) |
|---|---|---|
| Overall | ||
| 28-day mortality, n (%) | 52 (34%) | 78 (51%) |
| Mortality rate difference relative to control (95% CI) | -17.2 (-28.4, -2.6) | |
| p-Valueb | 0.0024 | |
| Baseline Viral Load | ||
| High viral load (CtNP ≤ 22)c | n=66 | n=64 |
| 28-day mortality, n (%) | 42 (64%) | 56 (88%) |
| Mortality rate difference relative to control (95% CI) | -23.9 (-43.8, -6.4) | |
| Low viral load (CtNP > 22)c | n=88 | n=88 |
| 28-day mortality, n (%) | 10 (11%) | 22 (25%) |
| Mortality rate difference relative to control (95% CI) | -13.6 (-31.8, -1.4) | |
a Both INMAZEB and Control were administered with optimized standard of care
b The result is significant according to the interim stopping boundary, p<0.028
c Cepheid GeneXpert Ebola® Assay used for detection of Zaire ebolavirus RNA
INMAZEB Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: INMAZEB worked similarly in males and females.
- Race: Data not collected.
- Age: INMAZEB worked similarly in all tested age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes primary efficacy results by sex and age subgroups.
Table 2. 28-Day Mortality Rates
| Subgroup | INMAZEB (N=154) |
Control (N=153) |
|---|---|---|
| Sex | ||
| Male | 21/64 (33%) | 31/73 (42%) |
| Female | 31/90 (34%) | 47/80 (59%) |
| Age group | ||
| < 6 years of age | 10/23 (43%) | 6/16 (38%) |
| 6 to < 12 years of age | 1/8 (13%) | 1/4 (25%) |
| 12 to < 18 years of age | 2/8 (25%) | 4/8 (50%) |
| Adults (age ≥18 years) | 39/115 (34%) | 67/125 (54%) |
INMAZEB Prescribing Information
What are the possible side effects?
INMAZEB may cause serious side effects including severe and life-threatening allergic reactions during and after the infusion.
The most common side effects of INMAZEB are fever, chills, fast heart rate, fast breathing and vomiting.
What are the possible side effects?
The table below summarizes adverse events that were reported during INMAZEB infusion. This table includes all patients who received either INMAZEB or the control throughout the trial.
Table 3. Adverse Events That Occurred during INMAZEB Infusion in ≥10% of Adult and Pediatric Subjects in the Trial
| Adverse Eventa | INMAZEB (N=154) % |
Controlc (N=168) % |
|---|---|---|
| Pyrexia (Elevation in fever) | 54 | 58 |
| Chills | 39 | 33 |
| Tachycardia | 20 | 32 |
| Tachypnea | 19 | 28 |
| Vomitingb | 19 | 23 |
| Hypotension | 15 | 31 |
| Diarrheab | 11 | 18 |
| Hypoxiab | 10 | 11 |
a Adverse events in this table were reported as preferred terms from a list of pre-defined or other adverse events that occurred on the day of infusion, and included signs and symptoms that occurred during or immediately after infusion
b Adverse events that were not pre-specified
c Investigational therapy administered as three separate infusions
INMAZEB Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: Data not collected.
- Age: The occurrence of side effects was similar in all tested groups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most frequent adverse events by sex and age subgroups. Demographic information for race was not collected.
Table 4. Subgroup Analysis of Adverse Events by Sex
| Adverse Event | INMAZEB N=154 |
Control N=168 |
||
|---|---|---|---|---|
| Male N=63 % |
Female N=91 % |
Male N=82 % |
Female N=86 % |
|
| Any adverse event | 78 | 78 | 90 | 87 |
| Pyrexia | 51 | 56 | 65 | 51 |
| Chills | 38 | 39 | 40 | 26 |
| Tachycardia | 22 | 19 | 27 | 36 |
| Tachypnea | 21 | 19 | 28 | 27 |
| Vomiting | 14 | 23 | 26 | 20 |
| Hypotension | 13 | 17 | 26 | 36 |
| Diarrhea | 14 | 9 | 22 | 15 |
Clinical Trial Data
Table 5. Subgroup Analysis of Adverse Events by Age
| INMAZEB N=154 |
Control N=168 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adverse Event | Age <6 N=24 % |
Age 6-11 N=7 % |
Age 12-17 N=8 % |
Age ≥18 N=115 % |
Age <6 N=19 % |
Age 6-11 N=5 % |
Age 12-17 N=8 % |
Age ≥18 N=136 % |
|
| Any adverse event | 79 | 86 | 63 | 78 | 84 | 80 | 100 | 89 | |
| Chills | 21 | 43 | - | 44 | 11 | 20 | 50 | 35 | |
| Pyrexia | 54 | 43 | 13 | 57 | 74 | 40 | 63 | 56 | |
| Tachycardia | 38 | - | - | 19 | 21 | 20 | 38 | 33 | |
| Tachypnea | 21 | 29 | - | 20 | 26 | 20 | 25 | 28 | |
| Vomiting | 21 | 14 | 25 | 19 | 21 | - | 38 | 23 | |
| Hypotension | 13 | 14 | 25 | 15 | 16 | 20 | 38 | 33 | |
| Diarrhea | 4 | 14 | 13 | 12 | 16 | 40 | 13 | 18 | |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved INMAZEB based primarily on evidence from a clinical trial (Trial 1/ NCT NCT03719586) of 322 patients with Zaire ebolavirus infection. The trial was conducted at four sites in the Democratic Republic of Congo during an outbreak that began in August 2018.
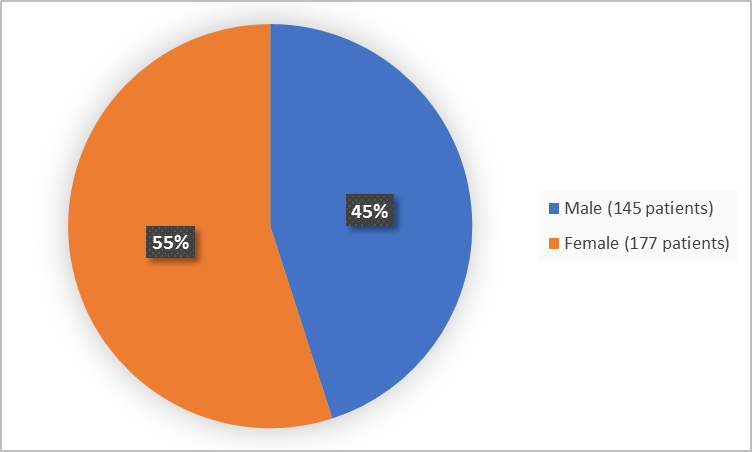
Figure 1 summarizes how many males and females were in the clinical trial.
Figure 1. Demographics by Sex (safety population)
Clinical Trial Data
Baseline Demographics by Race - data not collected.
Figure 2 summarizes the percentage of patients by age.
Figure 2. Demographics by Age (safety population)
Clinical Trial Data
Demographics by Ethnicity - data not collected.
Who participated in the trials?
The table below summarizes demographics of patients in the trial.
Table 6. Demographic Characteristics (safety population)
| Demographic Characteristic | INMAZEB N=154 |
Control N=168 |
TOTAL N=322 |
|---|---|---|---|
| Sex | |||
| Male | 63 (41 %) | 82 (49 %) | 145 (45 %) |
| Female | 91 (59 %) | 86 (51 %) | 177 (55 %) |
| Race (not collected) | |||
| Age (years) | |||
| Median | 26 | 27.5 | 27 |
| Min, Max | <1, 73 | <1, 70 | <1, 73 |
| Age Group | |||
| <6 years | 24 (16 %) | 19 (11 %) | 43 (13 %) |
| 6-11 years | 7 (4 %) | 5 (3 %) | 12 (4 %) |
| 12-17 years | 8 (5 %) | 8 (5 %) | 16 (5 %) |
| ≥18 years | 115 (75 %) | 136 (81 %) | 251 (78 %) |
| Ethnicity (not collected) | |||
| Geographic Region | |||
| US | 0 | 0 | 0 |
| Democratic Republic of Congo | 154 (100 %) | 168 (100 %) | 322 (100 %) |
Clinical Trial Data
How were the trials designed?
There was one trial that evaluated both, benefits and side effects of INMAZEB. The trial enrolled pediatric and adult patients (including pregnant women) with Zaire ebolavirus infection. All patients received standard, supportive care for the disease. In addition to the standard care, patients were randomly assigned to receive either a one-time dose of INMAZEB or one of the three other types of experimental treatments (including one as the control group). The patients and the health care providers knew which treatment was being given.
The benefit was evaluated by comparing the proportion of patients who died within 28 days from their trial enrollment in the INMAZEB-treated group to the patients who received the control treatment.
How were the trials designed?
The safety and efficacy of INMAZEB were established in a multi-center, open label, randomized active-controlled trial. The trial evaluated pediatric and adult patients (including pregnant women) who acquired Zaire ebolavirus infection. All patients received an optimized standard of care. In addition, patients were randomized to receive a single infusion of INMAZEB, an investigational control intravenously every third day, totaling 3 doses, or other investigational drug.
The primary efficacy endpoint was 28-day mortality. The primary analysis population included all subjects who were randomized and concurrently eligible to receive either INMAZEB or the investigational control during the same time period of the trial.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.