Drug Trials Snapshots: KRINTAFEL
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the KRINTAFEL Package Insert for complete information.
KRINTAFEL (tafenoquine)
Krin-tah-fell
GlaxoSmithKline
Approval date: July 20, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KRINTAFEL is a drug used to prevent relapse of malaria caused by the parasite, Plasmodium vivax. It is used in patients 16 years of age and older, who are already receiving medicine to treat acute Plasmodium vivax malaria infection.
Plasmodium vivax malaria is a serious disease of the blood that is spread by mosquitos infected by a parasite called Plasmodium vivax.
How is this drug used?
KRINTAFEL is administered as a single dose of 2 tablets taken by mouth.
What are the benefits of this drug?
More patients who received KRINTAFEL plus chloroquine (a drug approved for the treatment of acute malaria) were free of disease after 6 months in comparison to the patients who received placebo drug plus chloroquine.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the evaluated patients for Trial 1. The main trial endpoint was recurrence-free efficacy at 6 months after treatment.
Table 2. Recurrence-free Efficacy Rates of KRINTAFEL in Patients with P. vivax at 6 Months – Trial 1a
| KRINTAFEL/ Chloroquine (n = 260) | Chloroquine (n = 133) | |
|---|---|---|
| Recurrence-free efficacy | 155 (60%) | 35 (26%) |
| Recurrence | 85 (33%) | 88 (66%) |
| Missing/indeterminate outcome | 20 (8%) | 10 (8%) |
| ORb (95% CI) | 0.24 (0.15, 0.38) | |
| P value | > |
a All randomized patients were treated and had a positive parasite smear for P. vivax at baseline.
b Odds ratio of the risk of recurrence of KRINTAFEL plus chloroquine versus placebo plus chloroquine using logistic regression model with treatment and region as covariates. Subjects who did not demonstrate initial clearance took a concomitant medication with anti-malarial activity, or had a missing Day 180 assessment were considered ‘missing/indeterminate’ and were counted as recurrences in the analysis.
KRINTAFEL Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: KRINTAFEL worked similarly in males and females.
- Race: KRINTAFEL worked similarly in all tested races. The number of White patients was limited; therefore, differences in response among the White race and other tested races could not be determined.
- Age: The number of patients older than 65 years was limited. Therefore, differences among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex, race, and age.
Table 3. Trial 1: Recurrence-free Efficacy at 6 Months by Sex, Race, and Age (Intent -to -Treat population)
| Demographic Characteristics | KRINTAFEL + Chloroquine (N=260) | Chloroquine (N=133) |
|---|---|---|
| Sex | ||
| Male | 108/196 (55) | 23/97 (24) |
| Female | 47/64 (73) | 12/36 (33) |
| Race | ||
| White | 2/4 (50) | 0/3 (0) |
| Black or African American | 16/28 (57) | 3/14 (21) |
| Asian | 28/50 (56) | 8/26 (31) |
| American Indian or Alaska native | 53/81 (65) | 13/43 (30) |
| Multiple | 56/97 (58) | 11/47 (23) |
| Other | 2/4 (50) | 0/3 (0) |
| Age Group | ||
| 65> | 150/253 (59) | 34/131 (26) |
| ≥65 years | 5/7 (71) | 1/2 (50) |
FDA Review
What are the possible side effects?
KRINTAFEL may cause serious side effects including hemolytic anemia (a condition in which red blood cells are destroyed), methemoglobinemia (a blood disorder in which too little oxygen is delivered to cells), psychiatric reactions, and severe allergic reactions.
The most common side effects of KRINTAFEL are dizziness, nausea, vomiting, headache, and decreased hemoglobin (a substance in the blood that carries oxygen).
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with Plasmodium vivax malaria.
Table 4. Adverse Reactionsa Reported in ≥5% of Patients Receiving KRINTAFEL in a Randomized, Active-Controlled Trial (Trial 1)
| Adverse Reaction | KRINTAFEL + Chloroquine | Chloroquine |
|---|---|---|
| (n = 260) | (n = 133) | |
| % | % | |
| Dizziness | 8 | 3 |
| Nausea | 6 | 7 |
| Vomiting | 6 | 5 |
| Decreased Hemoglobin | 5 | 2 |
| Headache | 5 | 7 |
a Adverse reactions reported prior to Day 29 due to subsequent confounding by adverse events associated with recurrence of malaria or retreatment with another agent from the quinolone class.
KRINTAFEL Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar in all tested races. The number of White patients was limited; therefore, differences in occurrence of side effects among the White race and other tested races could not be determined.
- Age: The number of patients older than 65 years was limited. Therefore, differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most frequent adverse reaction, dizziness, by subgroup for the safety population.
Table 5. Pooled Subgroup Analysis of Dizziness (safety population)
| Demographic Characteristics | KRINTAFEL + Chloroquine n/N (%) | Chloroquine n/N (%) |
|---|---|---|
| Sex | ||
| Male | 14/196 (7) | 5/97 (5) |
| Female | 11/64 (17) | 6/36 (17) |
| Race | ||
| White | 0/4 (0) | 0/3 (0) |
| Black or African American | 2/28 (7) | 0/14 (0) |
| Asian | 1/50 (2) | 2/26 (8) |
| American Indian or Native Alaska | 22/81 (27) | 9/43 (21) |
| Other | 0/97 (0) | 0/47 (0) |
| Age Group | ||
| 65=""> | 22/253 (9) | 10/131 (8) |
| > 65 years | 3/7 (43) | 1/2 (50) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved KRITAFEL based primarily on evidence from a clinical trial (Trial 1/ NCT01376167) of 522 patients with Plasmodium vivax malaria. The trial was conducted at 8 sites in Asia, Africa, and Latin America.
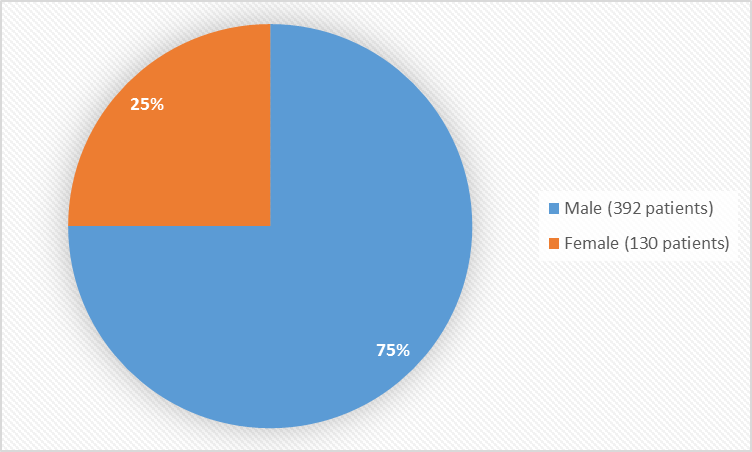
Figure 1 summarizes how many males and females were enrolled in the clinical trial used to evaluate efficacy and safety.
Figure 1. Baseline Demographics by Sex
FDA Review
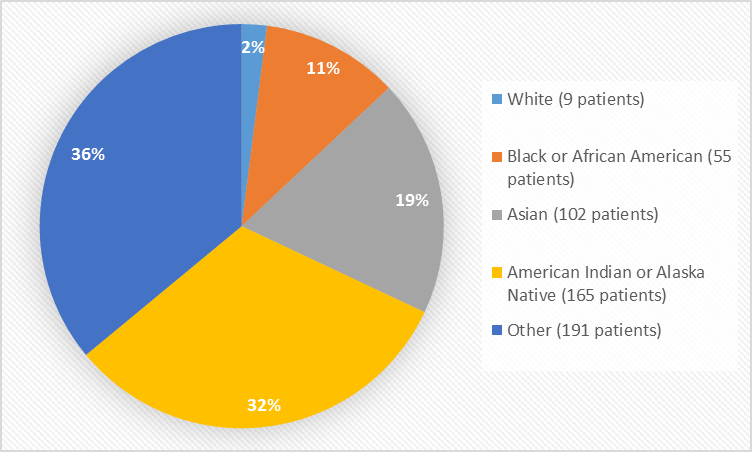
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trial used to evaluate efficacy and safety.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Trial by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 9 | 2% |
| Black or African American | 55 | 11% |
| Asian | 102 | 19% |
| American Indian or Alaska Native | 165 | 32% |
| Other | 191 | 36% |
FDA Review
Figure 3. Baseline Demographics by Age
Figure 3 summarizes the percentage of patients by age in in the clinical trial used to evaluate efficacy and safety.
Who participated in the trials?
The table below summarizes demographics of all patients in the trial.
Table 6. Demographic Characteristics
| Chloroquine +KRINTAFEL (N=260) | Chloroquine +Primaquine (N=129) | Chloroquine (N=133) | Total (N=522) | ||||
|---|---|---|---|---|---|---|---|
| Sex, n (%) | |||||||
| Male | 196 (75) | 99 (77) | 97 (73) | 392 (75) | |||
| Female | 64 (25) | 30 (23) | 36 (27) | 130 (25) | |||
| Race, n (%) | |||||||
| White | 4 (2) | 2 (2) | 3 (2) | 9 (2) | |||
| Black or African American | 28 (11) | 13 (10) | 14 (11) | 55 (11) | |||
| Asian | 50 (19) | 26 (20) | 26 (20) | 102 (19) | |||
| American Indian or Native Alaska | 81 (31) | 41 (32) | 43 (32) | 165 (32) | |||
| Other | 97 (37) | 47 (36) | 47 (35) | 191 (36) | |||
| Age in years | |||||||
| Mean (SD) | 35.0 (14.4) | 34.7 (14.3) | 35.3 (14.2) | 35.0 (14.3) | |||
| Median | 31.5 | 33.0 | 31.0 | 31.8 | |||
| Min, max | 15.0, 79.0 | 15.0, 66.0 | 17.0, 71.0 | 15.0, 79.0 | |||
| Age group, n (%) | |||||||
| 65=""> | 253 (97) | 126 (98) | 131 (98) | 510 (98) | |||
| ≥ 65 years | 7 (3) | 3 (2) | 2 (2) | 12 (2) | |||
| Ethnicity, n (%) | |||||||
| Hispanic or Latino | 182 (70) | 89 (69) | 93 (70) | 364 (70) | |||
| Not Hispanic or Latino | 78 (30) | 40 (31) | 40 (30) | 158 (30) | |||
| Country, n (%) | |||||||
| Brazil | 105 (40) | 52 (40) | 53 (40) | 210 (40) | |||
| Ethiopia | 28 (11) | 13 (10) | 14 (10) | 55 (10) | |||
| Cambodia | 19 (7) | 9 (7) | 10 (7) | 38 (7) | |||
| Peru | 77 (30) | 38 (29) | 40 (30) | 155 (30) | |||
| Philippines | 3 (1) | 2 (2) | 1 (1) | 69 (1) | |||
| Thailand | 28 (11) | 15 (12) | 15 (11) | (11) | |||
FDA Review
How were the trials designed?
Trial 1 was used to evaluate both, safety and benefits of KRINTAFEL. This trial enrolled patients with Plasmodium vivax malaria. All patients received a medicine used to treat acute malaria (chloroquine) for 3 days. In addition to chloroquine, patients were randomly assigned to receive either a one-time dose of KRINTAFEL (two 150-mg tablets) on Day 1 or Day 2, primaquine (a different medicine used to prevent relapse of malaria) or placebo. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
The benefit of KRINTAFEL was evaluated by comparing the number of patients who did not have a new Plasmodium vivax malaria infection 6 months after treatment with KRINTAFEL to the patients who received chloroquine alone.
How were the trials designed?
The safety and efficacy of KRINTAFEL were established in a randomized, double-blind, double-dummy, active - controlled trial that evaluated adult patients with acute Plasmodium vivax malaria. All patients received chloroquine phosphate (600 mg on Days 1 and 2, and 300mg on Day 3) to treat the acute infection. In addition to chloroquine phosphate, all patients received matching placebo and were randomized to receive either a one-time dose of KRINTAFEL (two 150-mg tablets) on Day 1 or Day 2, an active control (primaquine) on Days 2-15, or placebo for 15 days. The primary efficacy endpoint of the trial was the recurrence-free rate at 6 months.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.