Drug Trials Snapshots: LARTRUVO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to LARTRUVO Prescribing Information for complete information.
LARTRUVO (olaratumab)
(Lär – troo – vō)
Eli Lilly and Comp.
Approval date: October 19, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LARTRUVO is a drug used to treat a type of cancer called soft tissue sarcoma. It is to be used in adult patients together with another anti-cancer drug called doxorubicin when radiation or surgery is not possible.
Soft tissue sarcoma (STS) is a rare type of cancer that begins in the soft tissues of the body, such as muscles, tendons, fat, and blood vessels. There are more than 50 different types of STS.
How is this drug used?
LARTRUVO is given by a health care professional directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. LARTRUVO intravenous infusion is given over 60 minutes on Days 1 and 8 of each 21-day treatment cycle.
What are the benefits of this drug?
The patients who received LARTRUVO with doxorubicin lived longer than the patients who received doxorubicin alone. In the clinical trial that led to FDA approval, on average, patients taking LARTRUVO with doxorubicin lived 26.5 months and patients taking doxorubicin 14.7 months.
The length of time tumors did not grow after treatment with LARTRUVO with doxorubicin was on average 8.2 months compared to 4.4 months for patients who received doxorubicin alone.
The percent of patients with an objective response (tumor shrinkage) was 18.2 percent for patients who received LARTRUVO with doxorubicin and 7.5 percent for those who received doxorubicin alone. Given the small number of patients in the trial, there may be no difference in objective response rate between the two groups of patients.
More trials are ongoing to confirm and further assess the clinical benefit of LARTRUVO in different types of soft tissue sarcoma.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trial based on overall survival, progression free survival and objective response rate.
Table 2. Efficacy Results in Trial 1
| LARTRUVO + Doxorubicin N=66 | Doxorubicin N=67 | ||
|---|---|---|---|
| Overall Survival | |||
| Number of deaths (%) | 39 (59%) | 52 (78%) | |
| Median, months (95% CI) | 26.5 (20.9, 31.7) | 14.7 (9.2, 17.1) | |
| Hazard Ratio (95% CI)a | 0.52 (0.34, 0.79) | ||
| p-value | p> | ||
| Progression-Free Survivalb | |||
| Number of events (%) | 37 (56%) | 34 (51%) | |
| Median, months (95% CI) | 8.2 (5.5, 9.8) | 4.4 (3.1, 7.4) | |
| Hazard Ratio (95% CI)a | 0.74 (0.46, 1.19) | ||
| Objective Response Rate (CR + PR)b | |||
| (95% CI) | 18.2% (9.8, 29.6) | 7.5% (2.5, 16.6) | |
| CR, n (%) | 3 (4.5%) | 1 (1.5%) | |
| PR, n (%) | 9 (13.6%) | 4 (6%) | |
Abbreviations: CI = confidence interval, CR = complete response, PR = partial response
a Stratified Cox model
b Based on independent review.
LATRUVO Prescribing Information
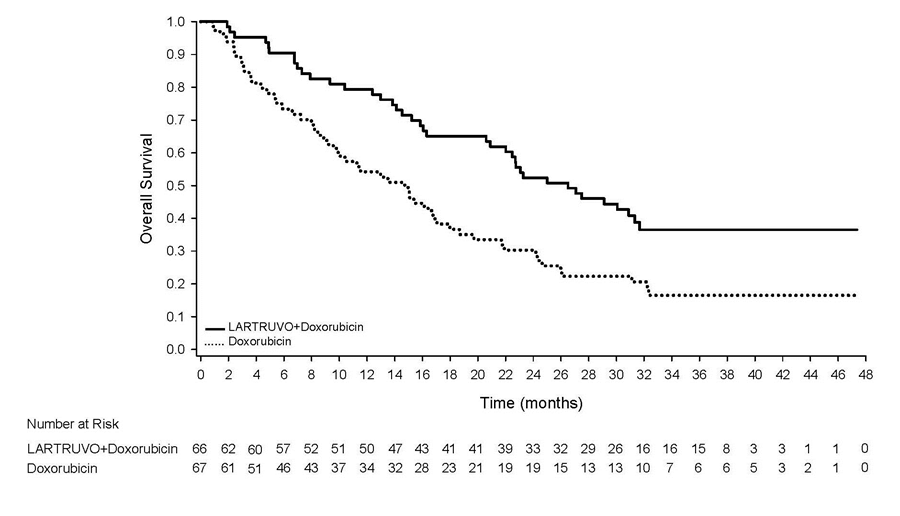
The figure below depicts efficacy results for the clinical trial based on overall survival.
Figure 4: Kaplan-Meier Curves of Overall Survival
LARTRUVO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: LARTRUVO worked similarly in men and women.
- Race: The majority of patients in the clinical trial were white. Differences in response to LARTRUVO among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in response to LARTRUVO between patients below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes Overall Survival (OS) efficacy results by subgroup.
Table 3. Subgroup Analysis of Overall Survival
| Sample Size (EVTS/N) | HR (95% CI) | |
|---|---|---|
| Overall | 39/66 : 52/67 | 0.52 (0.34, 0.79) |
| Age group | ||
| > | 30/48 : 33/43 | 0.54 (0.33, 0.89) |
| >65 | 9/18 : 19/24 | 0.48 (0.22, 1.07) |
| Sex | ||
| Men | 16/26 : 28/33 | 0.55 (0.3, 1.02) |
| Women | 23/40 : 24/34 | 0.53 (0.3, 0.94) |
| Race | ||
| Asian | 1/2 : 2/2 | 0.62 (0.05, 7) |
| Black Or African American | 3/6 : 3/5 | 0.5 (0.1, 2.49) |
| White | 32/55 : 47/60 | 0.52 (0.33, 0.81) |
EVTS=events, HR=hazard ratio, CI-confidence interval
FDA review
What are the possible side effects?
The most common side effects of LARTRUVO are nausea, fatigue, low levels of white blood cells, pain in muscles and joints, inflammation of the mucous membranes, hair loss, vomiting, diarrhea, decreased appetite, abdominal pain, nerve damage, and headache.
LARTRUVO may cause serious side effects, including infusion reactions and harm to an unborn baby.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in the clinical trial in ≥10% of patients in the LARTRUVO plus doxorubicin arm and at a higher Incidence than in the doxorubicin arm.
Table 4. Adverse Reactions Occurring in ≥10% (All Grades) of Patients in the LARTRUVO plus Doxorubicin Arm and at a Higher Incidence than in the Doxorubicin Arm (Between Arm Difference of ≥5% for All Grades or ≥2% for Grades 3 and 4) (Trial 1)
| Adverse Reactions | LARTRUVO plus Doxorubicin N=64 | Doxorubicin N=65 | |||||
|---|---|---|---|---|---|---|---|
| All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) | ||||
| Gastrointestinal Disorders | |||||||
| Nausea | 73 | 2 | 52 | 3 | |||
| Mucositis | 53 | 3 | 35 | 5 | |||
| Vomiting | 45 | 0 | 19 | 0 | |||
| Diarrhea | 34 | 3 | 23 | 0 | |||
| Abdominal Paina | 23 | 3 | 14 | 0 | |||
| General Disorders and Administrative Site Conditions | |||||||
| Fatigueb | 69 | 9 | 69 | 3 | |||
| Infusion-Related Reactions | 13 | 3 | 3 | 0 | |||
| Musculoskeletal and Connective Tissue Disorders | |||||||
| Musculoskeletal Painc | 64 | 8 | 25 | 2 | |||
| Skin and Subcutaneous Tissue Disorders | |||||||
| Alopecia | 52 | 0 | 40 | 0 | |||
| Metabolic and Nutritional Disorders | |||||||
| Decreased Appetite | 31 | 2 | 20 | 0 | |||
| Nervous System Disorders | |||||||
| Neuropathy | 22 | 0 | 11 | 0 | |||
| Headache | 20 | 0 | 9 | 0 | |||
| Psychiatric Disorder | |||||||
| Anxiety | 11 | 0 | 3 | 0 | |||
| Eye Disorder | |||||||
| Dry Eyes | 11 | 0 | 3 | 0 | |||
a Abdominal pain includes: abdominal pain, lower abdominal pain, and upper abdominal pain.
b Fatigue includes: asthenia and fatigue.
c Musculoskeletal pain includes: arthralgia, back pain, bone pain, flank pain, groin pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, muscle spasms, neck pain, and pain in extremity.
LARTRUVO Prescribing Information
The table below summarizes laboratory abnormalities in the clinical trial.
Table 5. Laboratory Abnormalities Worsening from Baseline in >10% (All Grades) of Patients in the LARTRUVO plus Doxorubicin Arm and Occurring at a Higher Incidence than in the Doxorubicin Arm (Between Arm Difference ≥5% for All Grades or ≥2% for Grades 3 and 4) (Trial 1)
| Laboratory Abnormality | LARTRUVO plus Doxorubicina | Doxorubicina | ||
|---|---|---|---|---|
| All Grades (%) | Grades 3-4 (%) | All Grades (%) | Grades 3-4 (%) | |
| Chemistry | ||||
| Hyperglycemia | 52 | 2 | 28 | 3 |
| Increased aPTTb | 33 | 5 | 13 | 0 |
| Hypokalemia | 21 | 8 | 15 | 3 |
| Hypophosphatemia | 21 | 5 | 7 | 3 |
| Increased Alkaline Phosphatase | 16 | 0 | 7 | 0 |
| Hypomagnesemia | 16 | 0 | 8 | 0 |
| Hematology | ||||
| Lymphopenia | 77 | 44 | 73 | 37 |
| Neutropenia | 65 | 48 | 63 | 38 |
| Thrombocytopenia | 63 | 6 | 44 | 11 |
a The incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement: LARTRUVO plus doxorubicin arm (range 60 to 63 patients) and doxorubicin arm (range 39 to 62 patients).
b aPTT = activated partial thromboplastin time
LARTRUVO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The risk of side effects was similar in men and women
- Race: The majority patients in the clinical trial were white. Differences in side effects among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Tables below summarize adverse events during the clinical trial by subgroup.
Table 6. Subgroup Analysis of Treatment-Emergent Adverse Events by Age–All Grades
| LARTRUVO + Doxorubicin N=64 | Single-agent Doxorubicin N=65 | |||
|---|---|---|---|---|
| 65> N=47 n (%) | ≥65 N=17 n (%) | 65> N=41 n (%) | ≥65 N=24 n (%) | |
| Any TEAE | 47 (100) | 16 (94.1) | 40 (97.6) | 24 (100) |
| Grade 3-4 TEAEs | 36 (76.6) | 15 (88.2) | 27 (65.9) | 18 (70.0) |
| Grade 5 TEAEs | 0 | 0 | 3 (73.1) | 6 (25.0) |
| Any SAE | 20 (42.6) | 7 (41.2) | 15 (36.6) | 10 (41.7) |
TEAE=treatment-emergent adverse event
SAE=serious adverse event
Table 7. Subgroup Analysis of Treatment-Emergent Adverse Events by Sex–All Grades
| LARTRUVO + Doxorubicin N=64 | Single-agent Doxorubicin N=65 | |||
|---|---|---|---|---|
| Men N=26 n (%) | Women N=38 n (%) | Men N=32 n (%) | Women N=33 n (%) | |
| Any TEAE | 22 (96.2) | 38 (100) | 32 (100) | 32 (97.0) |
| Grade 3-4 TEAEs | 19 (73.1) | 32 (84.2) | 21 (65.6) | 24 (72.7) |
| Grade 5 TEAEs | 0 | 0 | 8 (12.5) | 1 ( 3.0) |
| Any SAE | 8 (30.8) | 19 (50.0) | 12 (37.5) | 13 (39.4) |
TEAE=treatment-emergent adverse event
SAE=serious adverse event
Table 8. Subgroup Analysis of Treatment-Emergent Adverse Events by Race–All Grades
| LARTRUVO + Doxorubicin N=64 | Single-agent Doxorubicin N=65 | |||
|---|---|---|---|---|
| White N=53 n (%) | Non-White N=11 n (%) | White N=59 n (%) | Non-White N=6 n (%) | |
| Any TEAE | 52 (98.1) | 11 (100) | 58 (98.3) | 6 (100) |
| Grade 3-4 TEAEs | 43 (81.1) | 8 (72.7) | 42 (71.2) | 3 (50.0) |
| Grade 5 TEAEs | 0 | 0 | 8 (13.6) | 1 (16.7) |
| Any SAE | 2 (41.5) | 5 (45.5) | 23 (39.0) | 2 (33.3) |
TEAE=treatment-emergent adverse event
SAE=serious adverse event
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved LARTRUVO based primarily on the evidence from one clinical trial of 133 patients with advanced soft tissue sarcoma. The trial was conducted in the USA.
The figure below summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Clinical trial data
Figure 2 and Table 1 below summarize the percentage of patients by race enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Demographics of Trial by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 115 | 86 |
| Black or African American | 11 | 8 |
| Asian | 4 | 3 |
| Other | 3 | 2 |
Clinical trial data
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trial.
Table 9. Baseline Demographics of Patients in the Clinical Trial
| Demographic parameter | LARTRUVO + Doxorubicin N=66 n (%) | Doxorubicin N=67 n (%) | Total N=133 n (%) |
|---|---|---|---|
| Age | |||
| Mean (SD) | 56.8 (12.5) | 58.3 (12.5) | 57.6 (12.5) |
| Median (min-max) | 58.5 (22-85) | 58.0 (29-86) | 58 (22-86) |
| Age Group | |||
| 18-> | 48 (73) | 43 (64) | 91 (68) |
| ≥65 | 18 (27) | 24 (35) | 42(32) |
| Sex | |||
| Men | 26 (39) | 33 (49) | 59(44) |
| Women | 40 (61) | 34 (51) | 74 (56) |
| Race | |||
| White | 55 (83) | 60 (90) | 115 (86) |
| Black | 6 (9) | 5 (7) | 11 (8) |
| Asian | 2 (3) | 2 (3) | 4 (3) |
| Other | 3 (5) | 0 | 3 (2) |
| Country | |||
| U.S. | 66 (100) | 67 (100) | 133 (100) |
Clinical trial data
How were the trials designed?
The benefit and side effects of LARTRUVO were evaluated in one clinical trial of 133 patients with advanced soft tissue sarcoma. About half of the patients received LARTRUVO together with doxorubicin and half received only doxorubicin. The benefit of LARTRUVO was evaluated by measuring the length of time patients lived after treatment (overall survival), the length of time tumors did not grow after treatment (progression-free survival), and the percentage of patients who experienced shrinkage of their tumors (objective response rate).
How were the trials designed?
The safety and efficacy of LARTRUVO were established in single, open label, randomized, two-arm, active-controlled, multicenter clinical trial. Patients with advanced soft tissue sarcoma (STS) whose disease was not amenable to treatment with surgery or radiation, and who had not received prior anthracycline therapy were randomized to receive LARTRUVO + doxorubicin on day 1 and 8 of each 21-day cycle or doxorubicin alone on day 1 of each 21-day cycle.
Treatment continued for up to eight cycles or until disease progression or unacceptable treatment-related toxicity.
The efficacy outcome measures were overall survival (OS), and progression-free survival (PFS) and objective response rate (ORR) as assessed by investigator according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION