Drug Trials Snapshots: MOUNJARO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the MOUNJARO Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
MOUNJARO (tirzepatide)
mown-JAHR-OH
Eli Lilly and Company
Original Approval date: May 13, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MOUNJARO is a drug that that improves blood sugar control in adults with type 2 diabetes mellitus (DM) when used in addition to diet and exercise.
How is this drug used?
MOUNJARO is an injectable drug available in a prefilled pen. It is injected once weekly under the skin (subcutaneously) of the abdomen, thigh, or upper arm. MOUNJARO may be used alone or in combination with other FDA-approved diabetes medications such as metformin, sulfonylureas, sodium-glucose co-transporter 2 inhibitors, and insulin. In patients also using insulin injections, MOUNJARO and insulin should be injected separately and not mixed into the same syringe.
Who participated in the clinical trials?
The FDA approved MOUNJARO based on evidence from nine clinical trials of 7,769 patients with type 2 diabetes mellitus, of which 5,415 of these patients received MOUNJARO. The trials were conducted at 673 sites in 24 countries, including Argentina, Australia, Brazil, Canada, India, Israel, Japan, Mexico, Russian Federation, South Korea, Taiwan, multiple European countries, and the United States (including Puerto Rico). All nine trials were used to assess safety and five of these trials were used to assess the efficacy of MOUNJARO. The five trials used in the efficacy evaluation included 6,263 adult patients with type 2 diabetes mellitus. Four additional trials were included in the safety evaluation, for a total of 7,769 adult patients with type 2 diabetes; therefore, the number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety.
What are the benefits of this drug?
In patients with type 2 diabetes, treatment with MOUNJARO can lower HbA1c (hemoglobin A1c), which is a measure of blood sugar control.
What are the benefits of this drug (results of trials used to assess efficacy)?
The FDA considered the results of five global, randomized clinical trials to evaluate the efficacy of MOUNJARO in the treatment of adult patients with type 2 diabetes mellitus. In these trials, MOUNJARO was used as monotherapy, or in combination with oral antidiabetic medications with or without basal insulin, and compared to placebo, semaglutide, insulin degludec, and insulin glargine. The primary efficacy endpoint in each trial was change in HbA1c from baseline. The results of each trial are presented below in Table 1.
Table 1. Impact on HbA1c Change From Baseline in the Five Global Clinical Trialsa
| Trial | SURPASS-1 | SURPASS-2 | SURPASS-3 | SURPASS-4 | SURPASS-5 |
|---|---|---|---|---|---|
| mITT population (N)b | 475 | 1876 | 1435 | 1989 | 471 |
| Comparator | Placebo | Semaglutide 1 mg |
Insulin degludec | Insulin glargine | Placebo |
| Comparator-adjusted HbA1c change from baseline (95% Confidence Interval) | |||||
| MOUNJARO 5 mg | -1.7 (-2.0, -1.4) |
-0.2 (-0.3, -0.0) |
-0.6 (-0.7, -0.5) |
-0.7 (-0.9, -0.6) |
-1.2 (-1.5, -1.0) |
| MOUNJARO 10 mg | -1.6 (-1.9, -1.3) |
-0.4 (-0.5, -0.3) |
-0.8 (-0.9, -0.6) |
-0.9 (-1.1, -0.8) |
-1.5 (-1.8, -1.3) |
| MOUNJARO 15 mg | -1.6 (-1.9, -1.3) |
-0.5 (-0.6, -0.3) |
-0.9 (-1.0, -0.7) |
-1.0 (-1.2, -0.9) |
-1.5 (-1.7, -1.2) |
Source: Adapted from FDA review
a In the two placebo-controlled studies, MOUNJARO was used as monotherapy in SURPASS-1, and was used in combination with background insulin in SURPASS-5.
b The mITT (modified intent-to-treat) population consisted of all randomized patients who were exposed to at least one dose of study treatment. Patients who discontinued treatment due to failure to meet enrollment criteria were excluded.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: MOUNJARO worked similarly in females and males.
- Race: MOUNJARO worked similarly in American Indian/Alaska Natives, Asians, Black/African Americans, and Whites.
- Age: MOUNJARO worked similarly in patients younger than 65 years of age and in patients that are 65 years and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Differences between MOUNJARO and control for HbA1c percent change are presented according to sex, race, and age in Table 2, Table 3, and Table 4, respectively.
Table 2. Impact on HbA1c Change From Baseline (Comparator-Adjusted) With 95% Credible Intervals, by Sexa
| Trial (Comparator) |
Subgroup | MOUNJARO 5mg |
MOUNJARO 10 mg |
MOUNJARO 15 mg |
|---|---|---|---|---|
| SURPASS-1 (Placebo) |
Female | -1.6 (-1.9, -1.2) | -1.6 (-2.0, -1.2) | -1.5 (-1.9, -1.1) |
| Male | -1.7 (-2.1, -1.4) | -1.6 (-2.0, -1.3) | -1.6 (-2.0, -1.3) | |
| SURPASS-2 (Semaglutide) |
Female | -0.2 (-0.3, -0.0) | -0.4 (-0.6, -0.2) | -0.5 (-0.6, -0.3) |
| Male | -0.2 (-0.3, -0.0) | -0.4 (-0.6, -0.3) | -0.5 (-0.7, -0.4) | |
| SURPASS-3 (Insulin degludec) |
Female | -0.5 (-1.0, -0.1) | -0.7 (-1.2, -0.3) | -0.8 (-1.3, -0.4) |
| Male | -0.6 (-0.8, -0.3) | -0.7 (-1.0, -0.4) | -0.9 (-1.2, -0.5) | |
| SURPASS-4 (Insulin glargine) |
Female | -0.9 (-1.1, -0.7) | -1.1 (-1.3, -0.9) | -1.1 (-1.3, -0.9) |
| Male | -0.7 (-0.9, -0.6) | -0.8 (-1.0, -0.7) | -1.0 (-1.1, -0.8) | |
| SURPASS-5 (Placebo) |
Female | -1.3 (-1.6, -1.0) | -1.5 (-1.8, -1.2) | -1.5 (-1.9, -1.2) |
| Male | -1.2 (-1.5, -0.9) | -1.5 (-1.8, -1.3) | -1.4 (-1.7, -1.1) |
Source: FDA Statistical Review
a Estimates and credible intervals for treatment effect include relevance of outcomes from other subgroups.
Table 3. Impact on HbA1c Change From Baseline (Comparator-Adjusted) With 95% Credible Intervals, by Racea,b
| Trial (Comparator) |
Subgroup | MOUNJARO 5 mg |
MOUNJARO 10 mg |
MOUNJARO 15 mg |
|---|---|---|---|---|
| SURPASS-1 (Placebo) |
American Indian/ Alaska Native |
-1.6 (-2.0, -1.2) | -1.5 (-1.9, -1.1) | -1.7 (-2.1, -1.3) |
| Asian | -1.6 (-2.0, -1.3) | -1.6 (-2.0, -1.2) | -1.7 (-2.1, -1.3) | |
| White | -1.6 (-2.0, -1.3) | -1.5 (-1.9, -1.2) | -1.6 (-1.9, -1.2) | |
| SURPASS-2 (Semaglutide) |
American Indian/ Alaska Native |
-0.2 (-0.4, 0.2) | -0.3 (-0.6, 0.1) | -0.6 (-1.0, -0.3) |
| Asian | -0.2 (-0.7, 0.3) | -0.4 (-0.8, 0.2) | -0.6 (-1.3, -0.1) | |
| Black/African American | -0.2 (-0.7, 0.1) | -0.4 (-0.7, 0.1) | -0.6 (-1.1, -0.2) | |
| White | -0.2 (-0.3, -0.1) | -0.5 (-0.6, -0.3) | -0.4 (-0.6, -0.3) | |
| SURPASS-3 (Insulin degludec) |
Asian | -0.7 (-1.3, -0.3) | -0.8 (-1.3, -0.3) | -0.9 (-1.5, -0.5) |
| Black/ African American | -0.6 (-1.2, 0.0) | -0.7 (-1.3, -0.1) | -0.7 (-1.3, 0.2) | |
| White | -0.5 (-0.7, -0.4) | -0.7 (-1.0, -0.5) | -0.9 (-1.1, -0.6) | |
| SURPASS-4 (Insulin glargine) |
American Indian/ Alaska Native |
-0.8 (-1.1, -0.6) | -0.9 (-1.3, -0.6) | -1.0 (-1.2, -0.7) |
| Asian | -0.7 (-1.0, -0.4) | -1.0 (-1.4, -0.7) | -1.0 (-1.3, -0.6) | |
| Black/ African American | -0.8 (-1.2, -0.5) | -1.0 (-1.3, -0.6) | -1.0 (-1.3, -0.6) | |
| Multiple | -0.8 (-1.3, -0.5) | -1.0 (-1.4, -0.7) | -1.0 (-1.4, -0.6) | |
| White | -0.8 (-0.9, -0.6) | -0.9 (-1.1, -0.8) | -1.0 (-1.2, -0.9) | |
| SURPASS-5 (Placebo) |
Asian | -1.3 (-1.7, -0.9) | -1.4 (-1.8, -0.9) | -1.6 (-2.0, -1.3) |
| White | -1.2 (-1.5, -1.0) | -1.6 (-1.7, -1.3) | -1.5 (-1.8, -1.3) |
Source: FDA Statistical Review
a Estimates and credible intervals for treatment effect include relevance of outcomes from other subgroups.
b Due to insufficient sample sizes, the following race subgroups were excluded from the analyses in each trial: “Black/African American” in SURPASS-1; “Native Hawaiian/Other Pacific Island” and “Multiple” in SURPASS-2; “American Indian/Alaska Native” and “Native Hawaiian/Other Pacific Islander” in SURPASS-3; “Black/African American”, “American Indian/Alaska Native” and “Multiple” in SURPASS-5.
Table 4. Impact on HbA1c Change From Baseline (Comparator-Adjusted) With 95% Credible Intervals, by Agea
| Trial (Comparator) |
Subgroup | MOUNJARO 5 mg |
MOUNJARO 10 mg |
MOUNJARO 15 mg |
|---|---|---|---|---|
| SURPASS-1 (Placebo) |
<65 years | -1.7 (-2.0, -1.4) | -1.7 (-2.0, -1.3) | -1.7 (-2.1, -1.4) |
| ≥65 years | -1.6 (-2.0, -1.2) | -1.4 (-1.8, -0.8) | -1.0 (-1.7, -0.4) | |
| SURPASS-2 (Semaglutide) |
<65 years | -0.2 (-0.3, -0.0) | -0.4 (-0.5, -0.3) | -0.5 (-0.6, -0.4) |
| ≥65 years | -0.2 (-0.4, 0.1) | -0.4 (-0.6, -0.2) | -0.4 (-0.6, -0.2) | |
| SURPASS-3 (Insulin degludec) |
<65 years | -0.6 (-0.8, -0.3) | -0.8 (-1.1, -0.5) | -1.0 (-1.3, -0.7) |
| ≥65 years | -0.4 (-0.7, -0.1) | -0.5 (-0.8, -0.2) | -0.5 (-0.8, -0.1) | |
| SURPASS-4 (Insulin glargine) |
<65 years | -0.8 (-1.0, -0.6) | -1.0 (-1.2, -0.8) | -1.1 (-1.4, -1.0) |
| ≥65 years | -0.7 (-0.9, -0.5) | -0.8 (-1.0, -0.6) | -0.9 (-1.1, -0.6) | |
| SURPASS-5 (Placebo) |
<65 years | -1.3 (-1.7, -1.0) | -1.6 (-1.9, -1.3) | -1.6 (-1.9, -1.3) |
| ≥65 years | -1.1 (-1.5, -0.7) | -1.4 (-1.7, -1.0) | -1.2 (-1.6, -0.8) |
Source: FDA Statistical Review
a Estimates and credible intervals for treatment effect include relevance of outcomes from other subgroups.
What are the possible side effects?
MOUNJARO may cause serious side effects including inflammation of the pancreas (pancreatitis), low blood sugar, allergic reactions, kidney problems (kidney failure), severe stomach problems, and complications of diabetes-related eye disease (diabetic retinopathy). In studies with rats, MOUNJARO and medicines that work like MOUNJARO caused thyroid tumors, including thyroid cancer. It is not known if MOUNJARO will cause thyroid tumors, or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people.
The most common side effects in clinical trials included nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion (dyspepsia), and stomach (abdominal) pain.
What are the possible side effects?
Table 5 summarizes common adverse reactions (excluding hypoglycemia) from two placebo-controlled trials. MOUNJARO was used alone in one trial and in combination with basal insulin in the other trial.
Table 5. Adverse Reactions Reported in ≥5% of MOUNJARO-Treated Patients with Type 2 Diabetes Mellitus in Two Placebo-Controlled Trials
| Adverse Reaction | Placebo N=235 % |
MOUNJARO 5 mg N=237 % |
MOUNJARO 10 mg N=240 % |
MOUNJARO 15 mg N=241 % |
|---|---|---|---|---|
| Nausea | 4 | 12 | 15 | 18 |
| Diarrhea | 9 | 12 | 13 | 17 |
| Decreased appetite | 1 | 5 | 10 | 11 |
| Vomiting | 2 | 5 | 5 | 9 |
| Constipation | 1 | 6 | 6 | 7 |
| Dyspepsia | 3 | 8 | 8 | 5 |
| Abdominal pain | 4 | 6 | 5 | 5 |
Source: MOUNJARO Prescribing Information
Note: Percentages reflect the number of patients who reported at least 1 occurrence of the adverse reaction.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in females and males.
- Race: The occurrence of side effects was similar between American Indians/Alaska Natives, Asian, Black/African American, and White patients.
- Age: The occurrence of side effects was similar between patients younger or older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The frequency of gastrointestinal adverse reactions in the two placebo-controlled trials by sex, age, and race are shown in Table 6.
Table 6. Frequency of Gastrointestinal Adverse Reactions in Two Placebo-Controlled Trials by Sex, Race, and Age
| Demographic Parameter | Placebo N=235 n (%) |
MOUNJARO 5, 10, and 15 mg N=718 n (%) |
|---|---|---|
| Sex | ||
| Female | 29/113 (25.7) | 154/329 (46.8) |
| Male | 19/122 (15.6) | 134/389 (34.4) |
| Race | ||
| American Indian or Alaska Native | 4/26 (15.4) | 33/94 (35.1) |
| Asian | 8/60 (13.3) | 83/193 (43.0) |
| Black or African American | 0/5 (0.0) | 10/23 (43.5) |
| White | 36/143 (25.2) | 161/407 (39.6) |
| Age category | ||
| 18 to 64 years | 35/169 (20.7) | 200/487 (41.1) |
| ≥65 years | 13/66 (19.7) | 88/231 (38.1) |
Source: Adapted from FDA Review
DEMOGRAPHICS SNAPSHOT
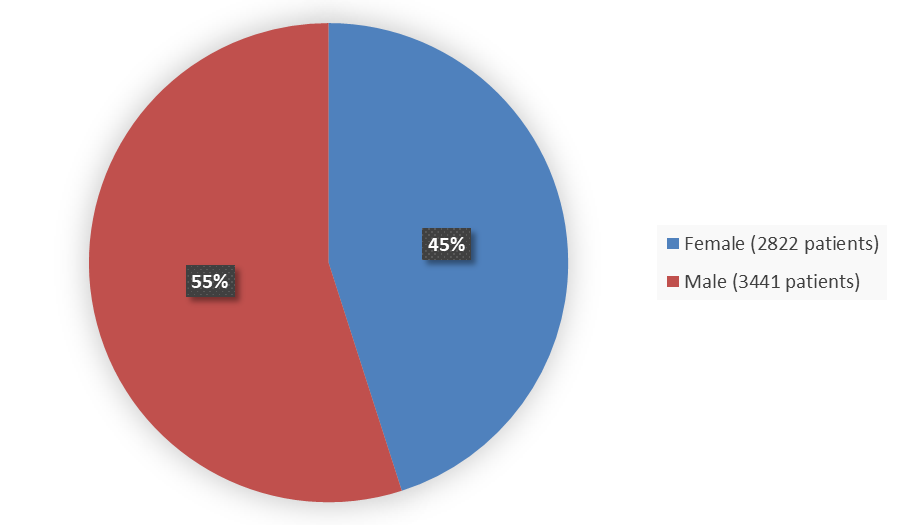
Figure 1 summarizes how many female and male patients were enrolled in the five combined clinical trials used to evaluate the efficacy of MOUNJARO.
Figure 1. Baseline Demographics of Five Global Efficacy Trials by Sex
Source: Adapted from FDA review
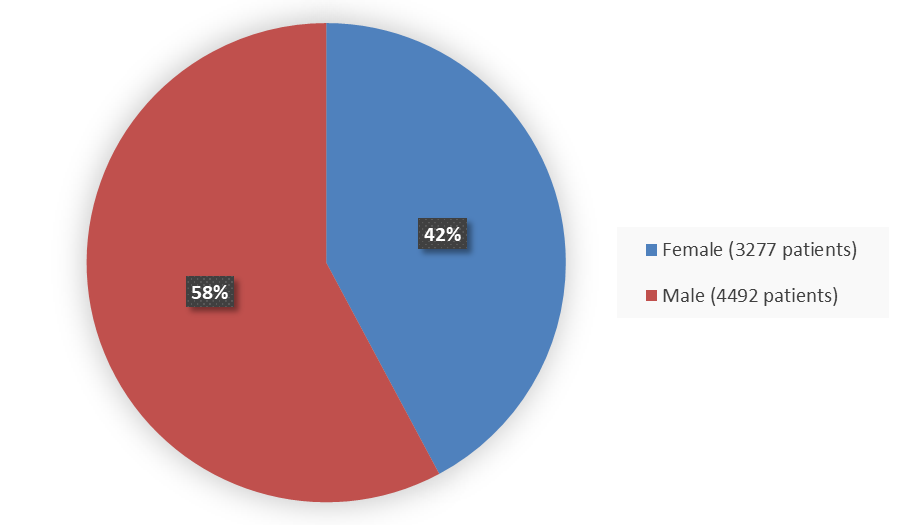
Figure 2 summarizes how many patients by sex were in the nine combined trials used to evaluate the side effects of MOUNJARO.
Figure 2. Baseline Demographics by Sex - Safety Population
Source: Adapted from FDA review
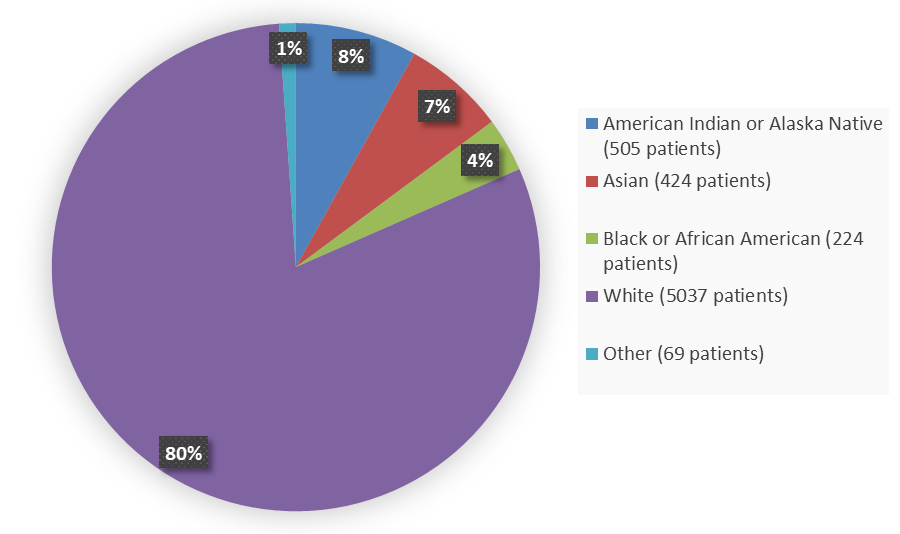
Figure 3 summarizes the percentage of patients by race enrolled in the five combined clinical trials used to evaluate the efficacy of MOUNJARO.
Figure 3. Baseline Demographics of Five Global Efficacy Trials by Race
Source: Adapted from FDA Review
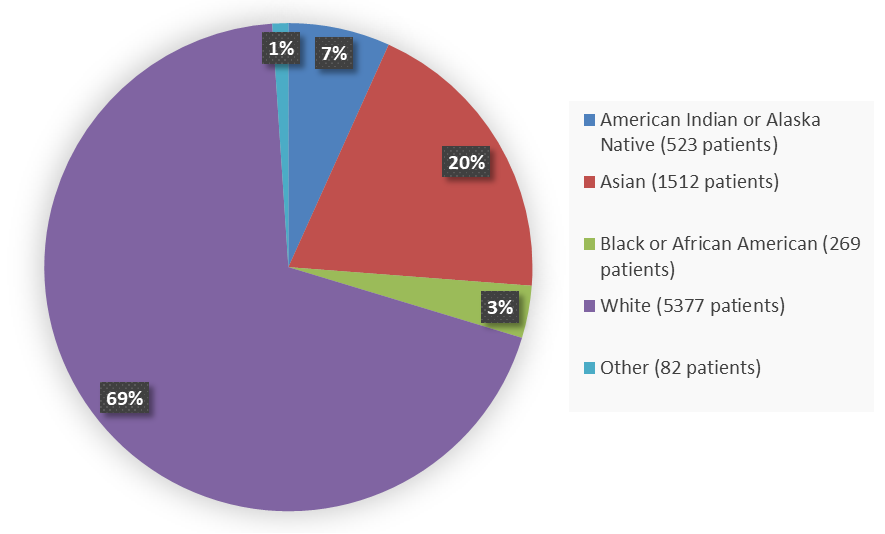
Figure 4 summarizes how many patients by race were in the nine combined trials used to evaluate the side effects of MOUNJARO.
Figure 4. Baseline Demographics by Race - Safety Population
Source: Adapted from FDA Review
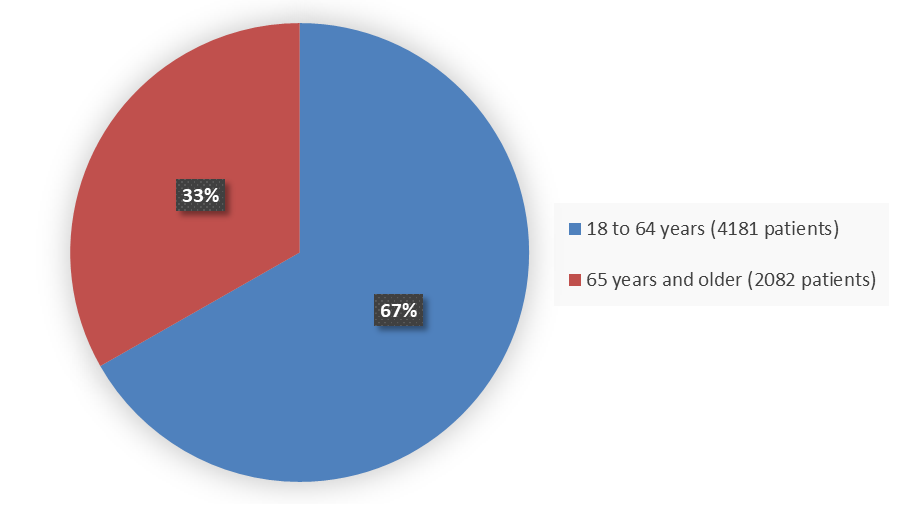
Figure 5 summarizes the percentage of patients by age enrolled in the five combined clinical trials used to evaluate the efficacy of MOUNJARO.
Figure 5. Baseline Demographics of Five Global Efficacy Trials by Age
Source: Adapted from FDA Review
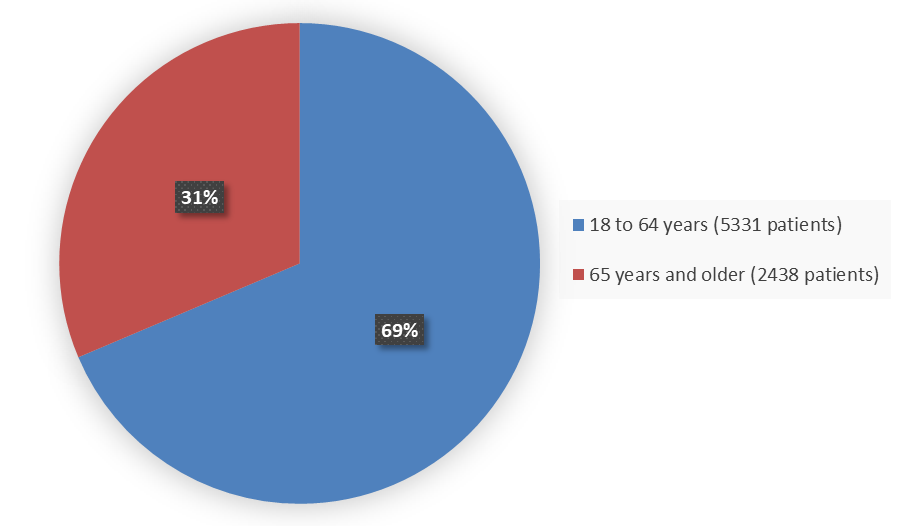
Figure 6 summarizes the percentage of patients by age enrolled in the nine combined trials used to evaluate the side effects of MOUNJARO.
Figure 6. Baseline Demographics by Age - Safety Population
Source: Adapted from FDA Review
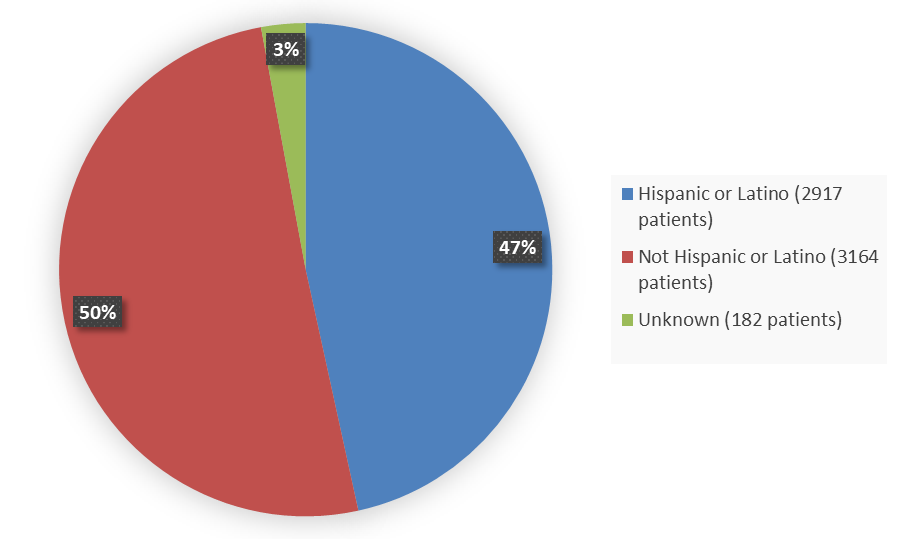
Figure 7 summarizes the percentage of patients by ethnicity enrolled in the five combined clinical trials used to evaluate the efficacy of MOUNJARO.
Figure 7. Baseline Demographics of Five Global Efficacy Trials by Ethnicity
Source: Adapted from FDA Review
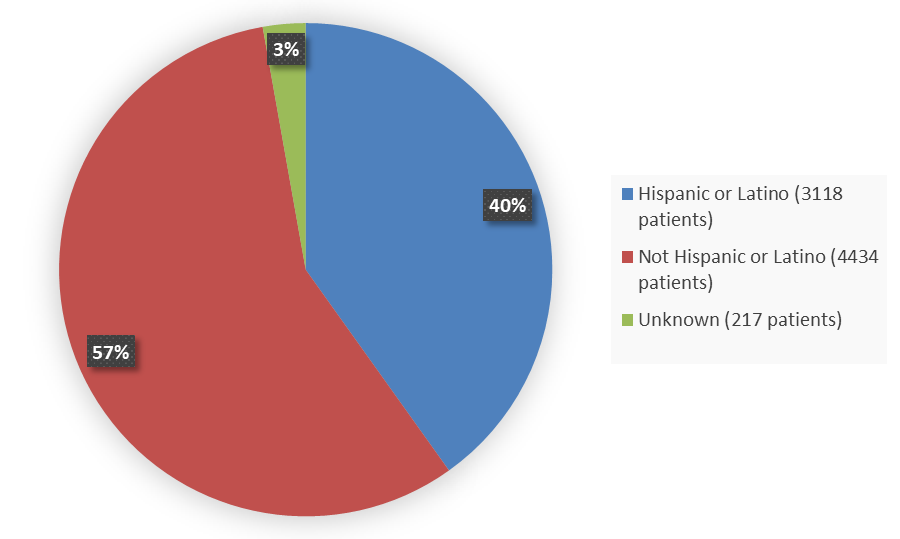
Figure 8 summarizes the percentage of patients by ethnicity enrolled in the nine combined trials used to evaluate the side effects of MOUNJARO.
Figure 8. Baseline Demographics by Ethnicity - Safety Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 7. Baseline Demographics - Efficacy Population
| Demographic Parameter | Number of Patients N=6263 |
Percentage |
|---|---|---|
| Sex | ||
| Female | 2822 | 45 |
| Male | 3441 | 55 |
| Race | ||
| American Indian or Native Alaskan | 505 | 8 |
| Asian | 424 | 7 |
| Black or African American | 224 | 4 |
| White | 5037 | 80 |
| Other | 69 | 1 |
| Unknown | 4 | 0 |
| Ethnicity | ||
| Hispanic or Latino | 2917 | 47 |
| Not Hispanic or Latino | 3164 | 50 |
| Unknown | 182 | 3 |
| Age category | ||
| 18 to 64 years | 4181 | 67 |
| ≥65 years | 2082 | 33 |
Source: Adapted from FDA Review
Table 8. Baseline Demographics - Safety Population
| Demographic Parameter | Number of Patients N=7769 |
Percentage |
|---|---|---|
| Sex | ||
| Female | 3277 | 42 |
| Male | 4492 | 58 |
| Race | ||
| American Indian or Native Alaskan | 523 | 7 |
| Asian | 1512 | 20 |
| Black or African American | 269 | 3 |
| White | 5377 | 69 |
| Other | 82 | 1 |
| Unknown | 6 | 0 |
| Ethnicity | ||
| Hispanic or Latino | 3118 | 40 |
| Not Hispanic or Latino | 4434 | 57 |
| Unknown | 217 | 3 |
| Age category | ||
| 18 to 64 years | 5331 | 69 |
| ≥65 years | 2438 | 31 |
Source: Adapted from FDA Review
How were the trials designed?
The benefits of MOUNJARO for the treatment of adult patients with type 2 diabetes mellitus were primarily evaluated in five clinical trials. In two of these trials (NCT #03954834 and NCT #04039503), patients were randomly assigned to receive either MOUNJARO or placebo injection weekly. Neither the patient nor the healthcare provider knew which treatment was being given until after the trials were completed. Treatment was given for 40 weeks. In the other three trials (NCT #3987919, 03882970, and 03730662), patients were randomly assigned to receive either MOUNJARO or another antidiabetic medication, and the patient and provider knew which medication was being given. Treatment was given for 40 weeks to 104 weeks. In each trial, HbA1c was measured from the start of the trial to the end of the trial and compared between the MOUNJARO group and the other groups.
The side effects of MOUNJARO were evaluated in the above five trials and in four additional trials that enrolled adult patients with type 2 diabetes mellitus. For three of the four trials (NCT #03131687, 03311724, and 03861052), neither the patient nor the healthcare provider knew which treatment was being given, and for the fourth trial (NCT #03861039) subjects only received MOUNJARO. Treatment was given for 12 weeks to 52 weeks.
How were the trials designed?
The efficacy of MOUNJARO was evaluated in five global clinical trials.
Two randomized, double-blind, placebo-controlled, multinational clinical trials evaluated the efficacy of three doses of MOUNJARO (5 mg, 10 mg, and 15 mg subcutaneously once weekly) as monotherapy or in combination with basal insulin. The primary endpoint was change in HbA1c from baseline to week 40.
Three additional randomized, active-controlled, multinational, non-inferiority design clinical trials evaluated the efficacy of the same three doses of MOUNJARO added to other antidiabetic medications compared to either semaglutide 1 mg subcutaneously once weekly, insulin degludec, or insulin glargine. These trials were open-label.
The safety of MOUNJARO was evaluated in the above five trials and four additional trials, of which two were conducted in Japan. For the Japanese trials, one was a randomized, double-blind, active-controlled trial that evaluated the effects of MOUNJARO as monotherapy, and the second was a randomized open-label trial without a control arm in which MOUNJARO was added to other antidiabetic medications. The two remaining trials were double-blind, placebo-controlled and placebo- and active-comparator controlled trials, respectively.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.