Drug Trials Snapshots: OJEMDA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the OJEMDA Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
OJEMDA (tovorafenib)
oh-JEM-dah
Day One Biopharmaceuticals Inc.
Original Approval date: April 23, 2024
DRUG TRIALS SNAPSHOT SUMMARY
What is the drug for?
OJEMDA is a kinase inhibitor that is used to treat patients 6 months of age and older with relapsed or refractory pediatric low-grade glioma (LGG) harboring a BRAF fusion or rearrangement or BRAF V600 mutation.
How is this drug used?
OJEMDA is administered as a tablet or oral suspension that is taken by mouth once weekly with or without food.
Who participated in the clinical trials?
The FDA approved OJEMDA based on evidence from one clinical trial (NCT04775485/FIREFLY-1) in patients 6 months to 25 years of age with previously treated solid tumors including pediatric LGG with changes in the BRAF gene. The trial was conducted at 32 sites in 11 countries including the United States, Australia, Canada, Denmark, Germany, Israel, Republic of Korea, Netherlands, Singapore, Switzerland, and the United Kingdom. Of the 137 patients with pediatric LGG participating in FIREFLY-1, 50 patients were treated in the United States and 87 patients were treated outside the United States. The demographics of the 50 patients treated in the United States were: median age was 8 years (range: 2 to 24); 62% male; 88% White, 4% Black or African American, 6% other race, 2% multiple races; and 8% Hispanic or Latino.
The safety of OJEMDA was evaluated in 137 patients with previously treated BRAF-mutant pediatric LGG who received at least one dose at the recommended phase 2 dose in FIREFLY-1. In the safety population, the demographics of the patients who received OJEMDA were: median age was 9 years (range: 1 to 24); 53% male; 58% White, 7% Asian, 2% Black or African American, 6% other races, 25% race was not reported; and 2.9% Hispanic or Latino. Some of these patients provided data for the assessment of the benefits of OJEMDA for BRAF-mutant pediatric LGG.
The efficacy of OJEMDA was evaluated in 76 patients with previously treated BRAF-mutant pediatric LGG. Demographics of the efficacy population were: median age was 8.5 years (range: 2 to 21); 53% male; 53% White, 7% Asian, 2.6% Black or African American, 3.9% multiple races, 8% other race, 26% where race was not reported; and 3.9% Hispanic or Latino.
How were the trials designed?
The benefits and side effects of OJEMDA were evaluated in FIREFLY-1, a single-arm clinical trial. The trial enrolled 137 patients with previously treated BRAF-mutant pediatric LGG who received OJEMDA once weekly.
The benefit of OJEMDA was evaluated by measuring the percentage of patients who had complete, partial, or minor shrinkage of their tumors (overall response rate or ORR) and by measuring the duration of that decrease in tumor size (duration of response or DOR).
How were the trials designed?
There was one multi-center, open-label, single-arm trial that provided data to evaluate the safety and efficacy of OJEMDA in patients with relapsed or refractory pediatric LGG harboring an activating BRAF alteration based on local laboratory testing. Patients were also required to have at least one measurable lesion as defined by Response Assessment in Neuro-Oncology (RANO) 2010 criteria. All patients had received at least one line of prior systemic therapy and had documented evidence of radiographic progression. Patients with tumors harboring additional activating molecular alteration(s) (e.g., IDH1/2 mutations, FGFR mutations, etc.) or patients with known or suspected diagnosis of neurofibromatosis type 1 were excluded.
Patients received OJEMDA approximately 420 mg/m2 orally once weekly (range: 290 to 476 mg/m2, 0.76 to 1.25 times the approved recommended dosage) according to body surface area, with a maximum dose of 600 mg, until disease progression or unacceptable toxicity.
The major efficacy outcome measure was ORR defined as the proportion of patients with complete response, partial response, or minor response by independent review based on RAPNO-LGG (Response Assessment in Pediatric Neuro-Oncology) criteria. Additional efficacy outcome measures were DOR, time to response, and ORR by independent review based on RANO-LGG (2011) criteria.
DEMOGRAPHICS SNAPSHOT
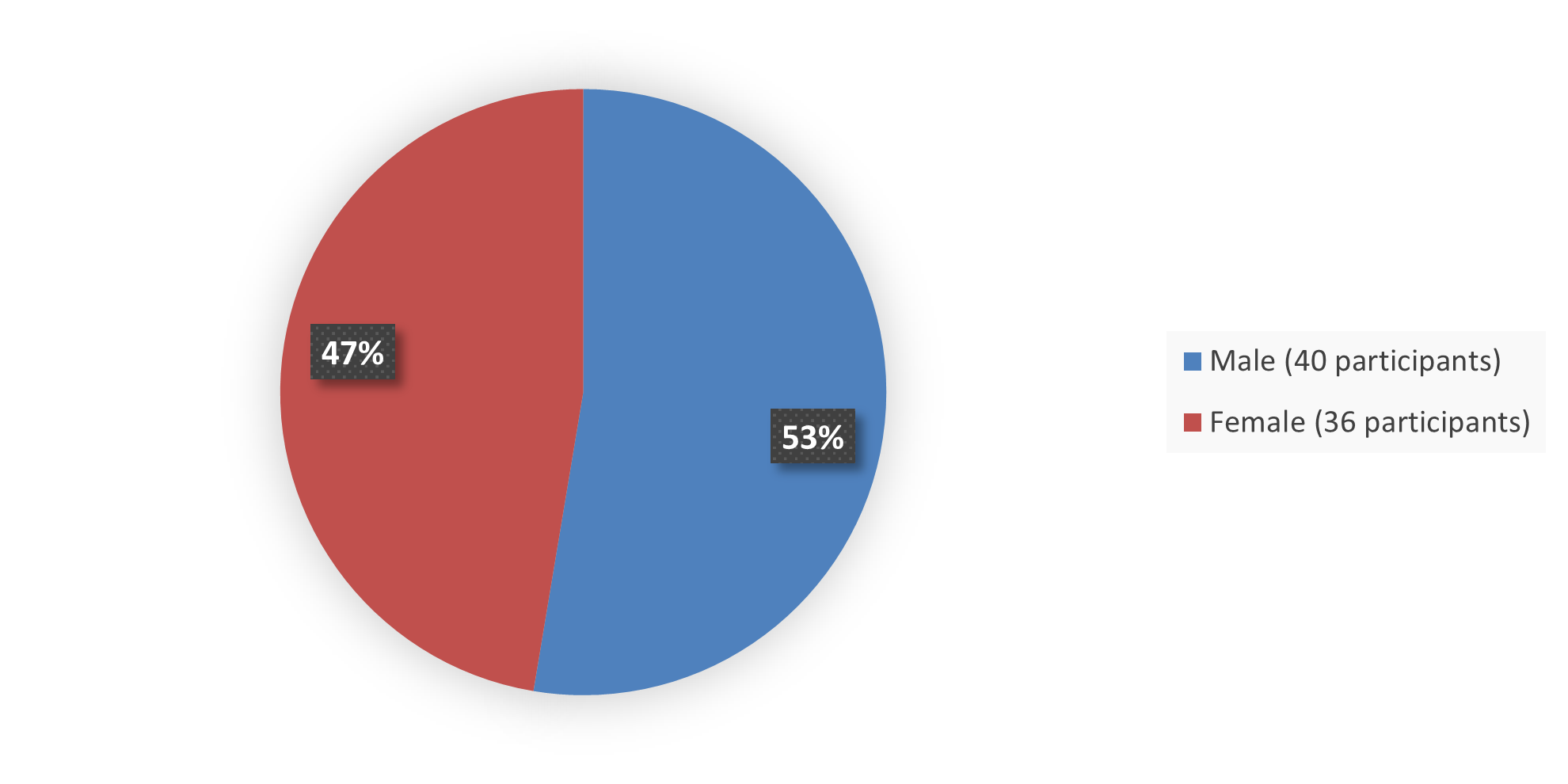
The primary efficacy population for this application included 76 patients with BRAF-mutant pediatric LGG. Figure 1 summarizes how many male and female patients were enrolled into the efficacy population in the clinical trial used to evaluate the safety and efficacy of OJEMDA.
Figure 1. Baseline Demographics by Sex Efficacy Population
Source: Adapted from FDA Review
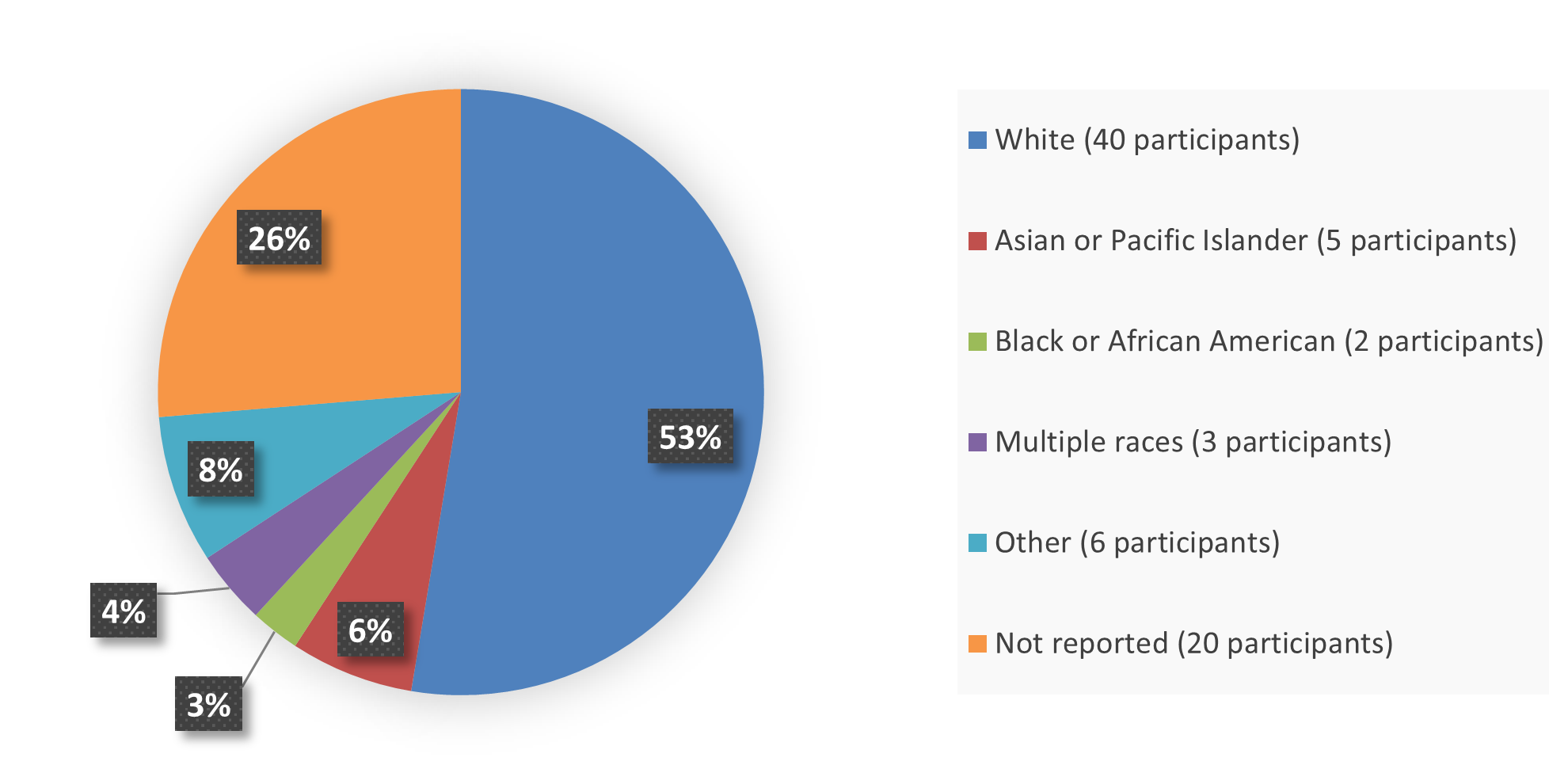
Figure 2 summarizes the percentage of patients by race in the efficacy population that enrolled into the clinical trial used to evaluate the safety and efficacy of OJEMDA.
Figure 2. Baseline Demographics by Race Efficacy Population
Source: Adapted from FDA Review
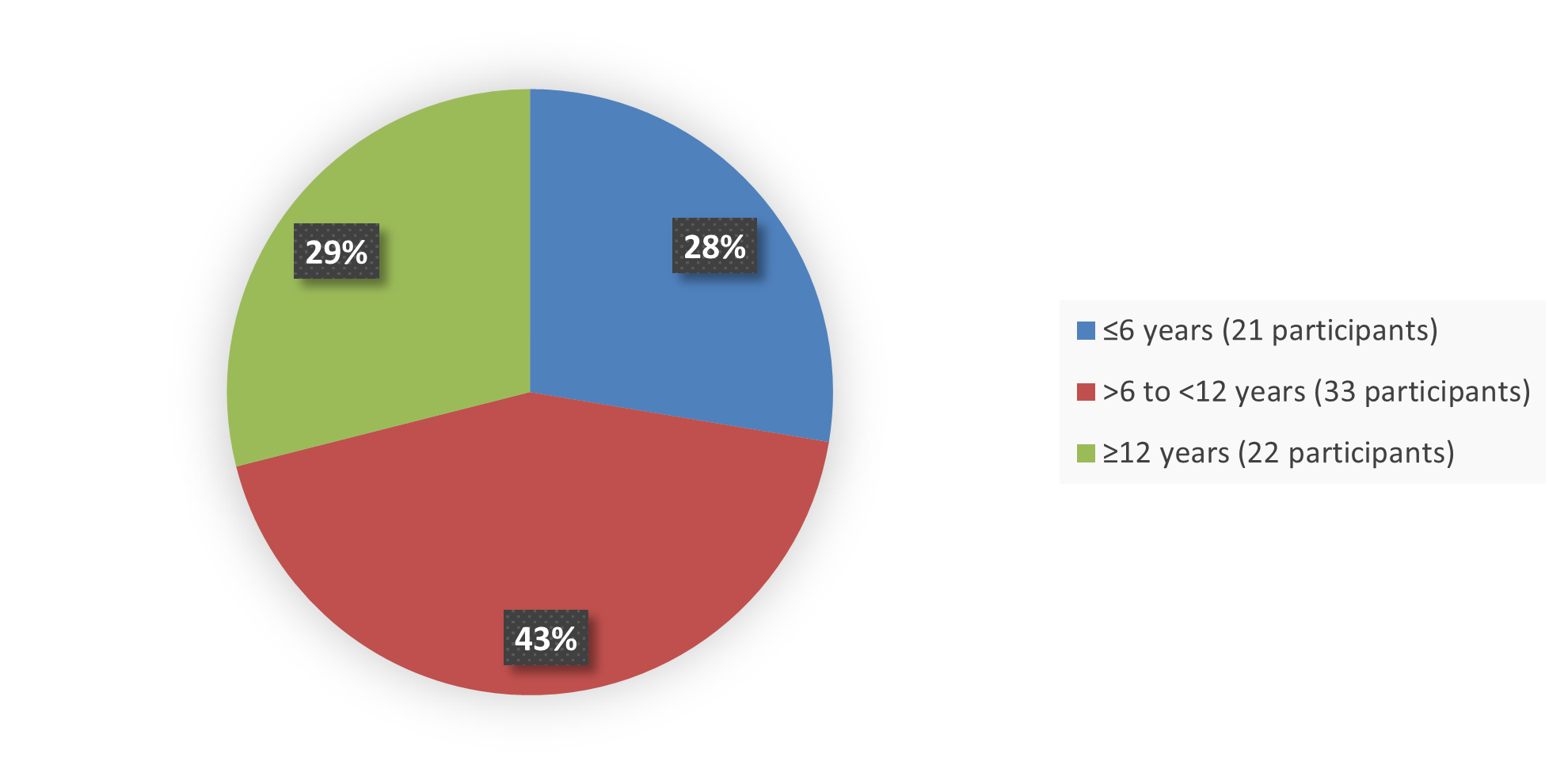
Figure 3 summarizes the percentage of patients by age in the efficacy population that enrolled into the clinical trial used to evaluate the safety and efficacy of OJEMDA.
Figure 3. Baseline Demographics by Age Efficacy Population
Source: Adapted from FDA Review
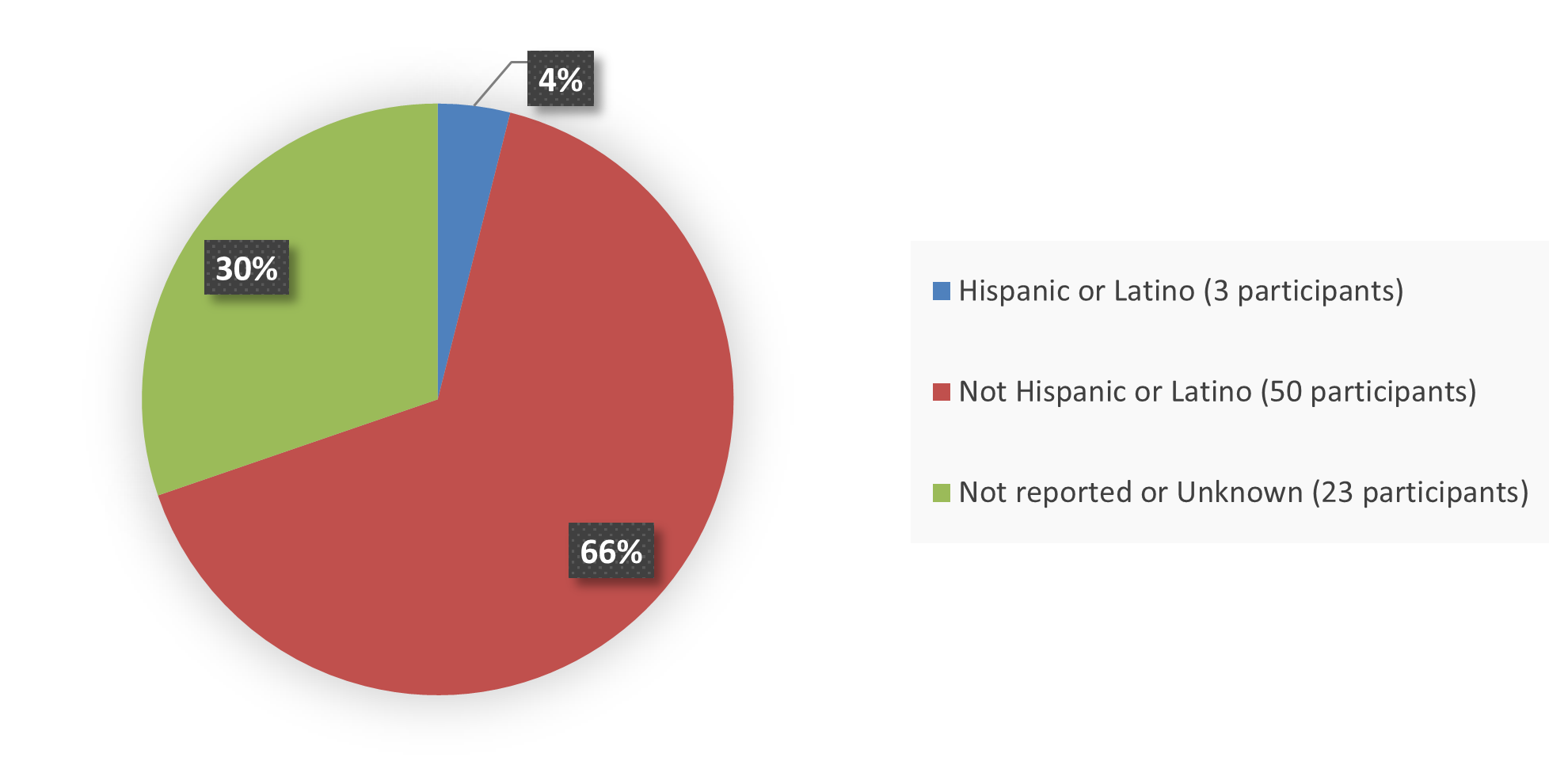
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics in FIREFLY-1 Efficacy Population
| Demographic |
OJEMDA |
| Age at baseline (years) | |
| Mean (SD) | 9.2 (4.0) |
| Median (min, max) | 8.5 (2, 21) |
| Sex n (%) | |
| Female | 36 (47) |
| Male | 40 (53) |
| Race n (%) | |
| White | 40 (53) |
| Asian | 5 (6) |
| Black or African American | 2 (3) |
| Multiple | 3 (4) |
| Other | 6 (8) |
| Not reported | 20 (26) |
| Ethnicity n (%) | |
| Hispanic or Latino | 3 (4) |
| Not Hispanic or Latino | 50 (66) |
| Not reported or unknown | 23 (30) |
| Country n (%) | |
| United States | 25 (33) |
| Outside the United States | 51 (67) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
OJEMDA was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well. In the FIREFLY-1 trial, 51% of patients with previously treated BRAF-mutant pediatric LGG experienced complete, partial, or minor shrinkage of their tumors; of these patients, 85% had shrinkage of their tumor that lasted more than six months.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes efficacy results for the indication based on ORR as determined by independent review according to RAPNO-LGG criteria. An additional efficacy outcome measure was DOR.
Table 2. Efficacy Results for Patients With Previously Treated BRAF-Mutant Pediatric LGG in FIREFLY-1 Efficacy Population
| RAPNO-LGG Efficacy Parameter |
OJEMDA |
| Overall response rate % (95% CI)a | 51 (40, 63) |
| Complete response n (%) | 0 (0) |
| Partial response n (%) | 28 (37) |
| Minor response n (%) | 11 (14) |
| Duration of response | N=39 |
| Median (95% CI)b, months | 13.8 (11.3, NE) |
| Duration of response ≥6 months % | 85 |
| Duration of response ≥12 months % | 23 |
Source: Adapted from OJEMDA Prescribing Information
* At least one measurable lesion at baseline based on RAPNO-LGG criteria.
a Based on Clopper-Pearson exact confidence interval.
b Based on Kaplan-Meier estimate.
Abbreviations: CI confidence interval; LGG low-grade glioma; NE not estimable; RAPNO Response Assessment in Pediatric Neuro-Oncology
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: OJEMDA worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore differences in how OJEMDA worked among races could not be determined.
- Age: OJEMDA worked similarly in patients ≤6 years of age, between >6 and <12 years of age, and ≥12 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes ORR by sex, race, age, and ethnicity subgroups. Results should be interpreted cautiously given the small number of patients in each subgroup.
Table 3. Efficacy Results by Sex, Race, Age, and Ethnicity for Patients With Previously Treated BRAF-Mutant Pediatric LGG in FIREFLY-1 Efficacy Population
| Demographic | OJEMDA N=76 n/Ns |
ORR (95% CI) |
| Sex | ||
| Female | 22/36 | 61 (43, 77) |
| Male | 17/40 | 42 (27, 59) |
| Race | ||
| White | 25/40 | 62 (46, 77) |
| Black or African American | 0/2 | NA |
| Asian | 3/5 | 60 (15, 95) |
| Other or not available | 8/26 | 31 (14, 52) |
| Age group (years) | ||
| ≤6 | 14/21 | 67 (43, 85) |
| >6 to <12 | 15/33 | 45 (28, 64) |
| ≥12 | 10/22 | 45 (24, 68) |
| Ethnicity | ||
| Hispanic or Latino | 0/3 | NA |
| Not Hispanic or Latino | 29/50 | 58 (43, 72) |
| Not reported or unknown | 10/23 | 43 (23, 66) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; LGG, low-grade glioma; N, number of patients in trial; n, number of responders; NA, not applicable; Ns, total number of patients for each specific subgroup; ORR, overall response rate
What are the possible side effects?
OJEMDA may cause serious side effects including bleeding problems, skin reactions including sensitivity to sunlight, liver problems, and slowed growth in children; based on studies in animals, neurofibromatosis type 1-associated tumors may increase in size when treated with OJEMDA.
The most common side effects of OJEMDA are rasha, hair color changes, tiredness, viral infection, vomiting, headache, fever, dry skin, constipation, nausea, acne, and upper respiratory tract infection.
What are the possible side effects (results of trials used to assess safety)?
Table 4 summarizes adverse reactions that occurred in patients with previously-treated pediatric LGG who received OJEMDA in FIREFLY-1.
Table 4. Adverse Reactions (≥20%) in Patients With Previously Treated BRAF-Mutant Pediatric LGG Who Received OJEMDA in FIREFLY-1 Safety Population
| OJEMDA N=137 |
|||
| Adverse Reaction | All Grades % | Grade 3 or 4 % | |
| Skin and subcutaneous tissue disorders | |||
| Rasha | 77 | 12 | |
| Hair color changes | 76 | 0 | |
| Dry skin | 36 | 0 | |
| Dermatitis acneiform | 31 | 1 | |
| Pruritus | 26 | 1 | |
| General disorders | |||
| Fatigue | 55 | 4 | |
| Pyrexia | 39 | 4 | |
| Edemab | 26 | 0 | |
| Infections and infestations | |||
| Viral infectionc | 55 | 7 | |
| Upper respiratory tract infection | 31 | 1.5 | |
| Paronychia | 26 | 1.5 | |
| Gastrointestinal disorders | |||
| Vomitingd | 50 | 4 | |
| Constipation | 33 | 0 | |
| Nausea | 33 | 0 | |
| Abdominal pain | 28 | 0 | |
| Diarrheae | 22 | 1.5 | |
| Stomatitisf | 20 | 0 | |
| Nervous system disorders | |||
| Headache | 45 | 1 | |
| Vascular disorders | |||
| Hemorrhageg | 42 | 5* | |
Source: Adapted from OJEMDA Prescribing Information
a Includes terms erythema multiforme, eczema, rash erythematous, rash macular, rash follicular, rash pruritic, rash maculopapular, rash, rash popular, rash pustular, skin exfoliation, drug eruption, dermatitis, and dermatitis bullous.
b Includes terms lip edema, periorbital edema, edema peripheral, localized edema, face edema, and vulval edema.
c Includes terms viral infection, rhinovirus infection, enterovirus infection, viral upper respiratory tract infection, enterocolitis viral, oral herpes, gastroenteritis viral, influenza, influenza like illness, respiratory syncytial virus infection, enterovirus infection, coronavirus infection, COVID-19, SARS-COV-2 test positive, herpes simplex, parainfluenza virus infection, adenoviral upper respiratory infection, viraemia, adenovirus infection, conjunctivitis viral, eye infection viral, metapneumovirus infection, parvovirus infection, respiratory syncytial virus bronchiolitis, respiratory tract infection viral, viral pharyngitis, viral rhinitis, and viral tonsillitis.
d Includes terms retching and hematemesis.
e Includes terms colitis and enterocolitis.
f Includes terms mouth ulceration, mucosal inflammation, aphthous ulcer, and cheilitis.
g Includes terms tumor hemorrhage, gastrointestinal hemorrhage, subdural hemorrhage, epistaxis, intracranial tumor hemorrhage, upper gastrointestinal hemorrhage, lower gastrointestinal hemorrhage, vaginal hemorrhage, gingival bleeding, post procedural hemorrhage, hemoptysis, and anal hemorrhage.
* Includes one Grade 5 event.
Abbreviations: LGG, low-grade glioma; SARS, severe acute respiratory syndrome
Table 5 summarizes select laboratory abnormalities that occurred in patients with previously treated pediatric LGG who received OJEMDA in FIREFLY-1.
Table 5. Select Laboratory Abnormalities (≥20%) That Worsened From Baseline in Patients With Previously Treated BRAF-Mutant Pediatric LGG Who Received OJEMDA in FIREFLY-1
| OJEMDA2 N=137 |
||
| Laboratory Abnormality1 | All Grades % | Grade 3 or 4 % |
| Hematology | ||
| Decreased hemoglobin | 90 | 15 |
| Decreased lymphocytes | 50 | 2 |
| Decreased leukocytes | 31 | 2 |
| Increased lymphocytes | 23 | 0 |
| Chemistry | ||
| Decreased phosphate | 87 | 25 |
| Increased AST | 83 | 2 |
| Increased creatine phosphokinase | 83 | 11 |
| Increased LDH | 73 | 0 |
| Decreased potassium | 51 | 2 |
| Increased ALT | 50 | 5 |
| Increased bilirubin | 22 | 1 |
| Decreased albumin | 24 | 5 |
| Decreased sodium | 20 | 2 |
Source: Adapted from FDA Review
1 Severity as defined by National Cancer Institute CTCAE v5.0
2 The denominator for each laboratory parameter is based on the number of patients with a baseline and post-treatment laboratory value available which ranged from 67 to 137 patients.
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore differences in the occurrence of side effects could not be determined.
- Age: There were no major differences in safety in patients based on age; however, there were fewer patients younger than 6 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6, Table 7, and Table 8 summarize adverse reactions that occurred by sex, race and age subgroups.
Table 6. Side Effects by Sex for Patients With Previously Treated BRAF-Mutant Who Received OJEMDA in FIREFLY-1 Safety Population
| Side Effect | Males N=73 % |
Females N=64 % |
| TEAEs | 100 | 100 |
| TEAEs grade ≥3 | 66 | 59 |
| Serious TEAEs | 47 | 42 |
| Fatal TEAEs | 1.4 | 0 |
Source: Adapted from FDA Review
Abbreviations: LGG, low-grade glioma; TEAE, treatment-emergent adverse event
Table 7. Side Effects by Race for Patients With Previously Treated BRAF-Mutant Pediatric LGG Who Received OJEMDA in FIREFLY-1 Safety Population
| Side Effect | White N=79 % |
Asian N=10 % |
Black or African American N=9 % |
Multiple Races N=3 % |
Other N=8 % |
Not Reported N=34 % |
| TEAEs | 100 | 100 | 100 | 100 | 100 | 100 |
| TEAEs grade ≥3 | 58 | 40 | 100 | 100 | 88 | 68 |
| Serious TEAEs | 41 | 20 | 67 | 67 | 88 | 47 |
| Fatal TEAEs | 1.3 | 0 | 0 | 0 | 0 | 0 |
Source: Adapted from FDA Review
Abbreviations: LGG, low-grade glioma; TEAE, treatment-emergent adverse event
Table 8. Side Effects by Age for Patients With Previously Treated BRAF-Mutant Pediatric LGG Who Received OJEMDA in FIREFLY-1 Safety Population
| Side Effect | 6 Months to <6 Years N=30 % |
6 to <12 Years N=65 % |
12 to ≤25 Years N=42 % |
| TEAEs | 100 | 100 | 100 |
| TEAEs grade ≥3 | 77 | 62 | 55 |
| Serious TEAEs | 70 | 43 | 29 |
| Fatal TEAEs | 0 | 0 | 2.4 |
Source: Adapted from FDA Review
Abbreviations: LGG, low-grade glioma; TEAE, treatment-emergent adverse event
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as and is given the same way as an active drug