Drug Trials Snapshots: ORLYNVAH
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ORLYNVAH Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ORLYNVAH (sulopenem etzadroxil and probenecid)
or lin’ vah
Iterum Therapeutics US Limited
Original Approval date: October 25, 2024
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ORLYNVAH is a combination of sulopenem etzadroxil (a penem antibiotic drug) and probenecid (a kidney transport blocking drug) that is indicated for the treatment of uncomplicated urinary tract infections (uUTI) caused by the bacteria Escherichia coli, Klebsiella pneumoniae, or Proteus mirabilis in adult female patients who have limited or no alternative oral antibiotic treatment options.
How is this drug used?
ORLYNVAH is a tablet that is taken two times each day for five days.
Who participated in the clinical trials?
The FDA approved ORLYNVAH based on evidence from two clinical trials that included 3,861 adult females with uUTI. The trials were conducted at 246 sites in the United States, Russia, and Ukraine. The majority of patients were enrolled in the United States while 700 patients were enrolled in Russia and Ukraine. The average age of patients from the United States was 48 years, with ages ranging from 18 to 96 years. Patients from the United States were White (78.3%), Black or African American (17.3%), or of other races (4.4%).
How were the trials designed?
The benefit (efficacy) and safety of ORLYNVAH were evaluated in two clinical trials of adult females with uUTI. Patients were enrolled in the trials if they met certain criteria and had at least two of the following symptoms: pain or burning while urinating, urinating frequently, needing to urinate urgently, or pain in the lower abdomen. However, despite having symptoms, not all patients had growth of a bacteria on culture of their urine sample. In the clinical trials, a diagnosis of uUTI was made in patients who had the symptoms listed above and growth of a bacteria on urine culture (positive culture). In the two trials, adult females with uUTI were chosen at random to receive either ORLYNVAH or a comparator antibiotic (the two trials used different comparator antibiotics). Neither the patient nor the investigator knew which treatment was given. The benefit of ORLYNVAH was measured only in patients who had urinary symptoms and a positive urine culture before treatment (efficacy population) by comparing the percentage of patients treated with ORLYNVAH versus the comparator antibiotic who experienced cure of their uUTI-related symptoms and had a negative urine culture after treatment.
How were the trials designed?
Trial 1 was a randomized, double-blind clinical trial that included 2,222 adult females with uUTI who were randomized. A total of 2,214 patients received either oral ORLYNVAH (sulopenem etzadroxil 500 mg and probenecid 500 mg) twice daily for five days or oral amoxicillin/clavulanate 875 mg/125 mg twice daily for five days.
The efficacy population, which included all patients who had at least one uropathogen isolated at baseline (≥105 CFU/mL) and received at least one dose of study drug, consisted of 990 patients.
Composite response (combined microbiological response and clinical cure rates), the primary efficacy endpoint, was determined by comparing the response rate of ORLYNVAH to amoxicillin/clavulanate at the test-of-cure (TOC) visit (12 days after randomization) in the efficacy population as well as in two sub-populations: (1) efficacy population with baseline uropathogens susceptible to amoxicillin/clavulanate (minimum inhibitory concentration [MIC] ≤8/4 µg/mL); and (2) efficacy population with baseline uropathogens non-susceptible to amoxicillin/clavulanate (MIC ≥16/8 µg/mL). Clinical cure was defined as the resolution of patient-reported uUTI symptoms and no new uUTI symptoms. Microbiological response was defined as a reduction of all baseline uropathogens to less than 103 CFU/mL in the urine.
Trial 2 was a multinational randomized, double-blind clinical trial that included 1,660 adult females with uUTI who were randomized and received either oral ORLYNVAH (sulopenem etzadroxil 500 mg and probenecid 500 mg) twice daily for five days or oral ciprofloxacin 250 mg twice daily for three days.
The efficacy population, which included all patients who had at least one uropathogen isolated at baseline (≥105 CFU/mL), consisted of 1,105 patients.
Composite response (combined microbiological response and clinical cure), the primary efficacy endpoint, was determined by comparing the response rate of ORLYNVAH to ciprofloxacin at the TOC visit (12 days after randomization) in two primary populations: (1) efficacy population with baseline uropathogens susceptible to ciprofloxacin (MIC ≤1 µg/mL); and (2) efficacy population with baseline uropathogens non-susceptible to ciprofloxacin (MIC ≥2 µg/mL). Clinical cure was defined as the resolution of patient-reported uUTI symptoms and no new uUTI symptoms. Microbiological response was defined as a reduction of all baseline uropathogens to less than 103 CFU/mL in the urine.
DEMOGRAPHICS SNAPSHOT
The demographics and benefit (efficacy) data include the study participants who had both uUTI symptoms and a positive urine culture before treatment and who received at least one dose of ORLYNVAH or comparator antibiotic.
All patients (100%) enrolled in the two clinical trials were female.
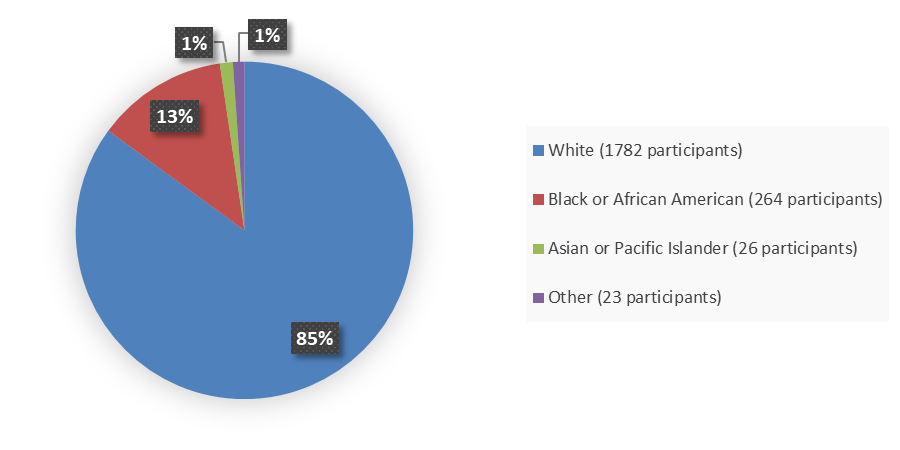
Figure 1 summarizes how many patients by race were enrolled in the combined clinical trials used to evaluate the efficacy of ORLYNVAH.
Figure 1. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
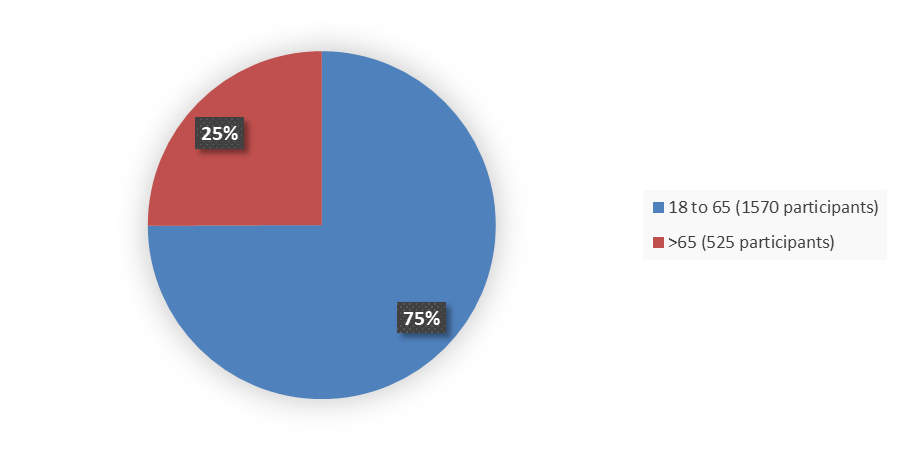
Figure 2 summarizes how many patients by age were enrolled in the combined clinical trials used to evaluate the efficacy of ORLYNVAH.
Figure 2. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
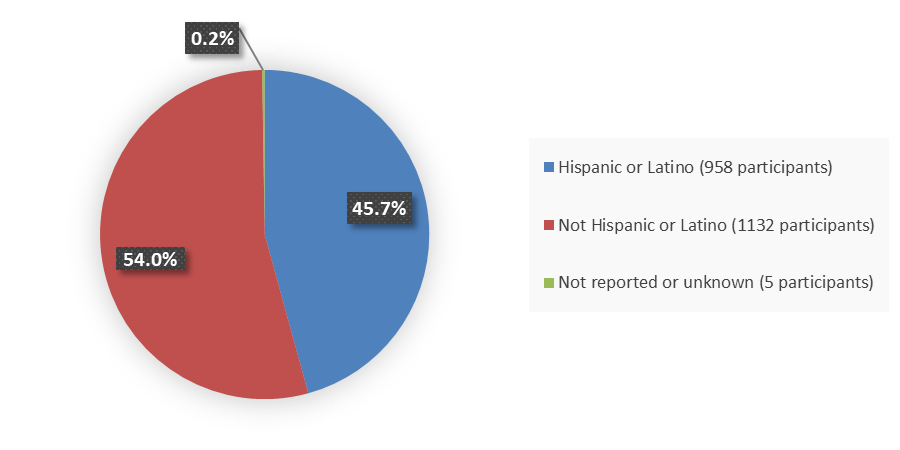
Figure 3 summarizes how many patients by ethnicity were enrolled in the combined clinical trials used to evaluate the efficacy of ORLYNVAH.
Figure 3. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics, Efficacy Population
| Demographic | Trial 1 | Trial 2 | ||
|---|---|---|---|---|
ORLYNVAH | Amoxicillin/ | ORLYNVAH | Ciprofloxacin | |
| Sex, n (%) | ||||
| Female | 522 (100) | 468 (100) | 538 (100) | 567 (100) |
| Age, years | ||||
| Mean (SD) | 50.3 (17.3) | 48.6 (17.2) | 51.8 (19.1) | 51.5 (19.1) |
| Median (min, max) | 52 (18, 91) | 50 (18, 93) | 54 (18, 89) | 52 (18, 96) |
| Age group, years, n (%) | ||||
| ≤65 | 409 (78.4) | 376 (80.3) | 381 (70.8) | 404 (71.3) |
| >65 | 113 (21.6) | 92 (19.7) | 157 (29.2) | 163 (28.7) |
| Race, n (%) | ||||
| White | 419 (80.3) | 370 (79.1) | 478 (88.8) | 515 (90.8) |
| American Indian or Alaska Native | 1 (<1) | 1 (<1) | 4 (<1) | 0 |
| Asian | 10 (1.9) | 8 (1.7) | 5 (<1) | 3 (<1) |
| Black or African American | 84 (16.1) | 84 (17.9) | 50 (9.3) | 46 (8.1) |
| Native Hawaiian or other Pacific Islander | 0 | 1 (<1) | 0 | 0 |
| Other | 8 (1.5) | 4 (<1) | 1 (<1) | 3 (<1) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 333 (63.8) | 296 (63.2) | 162 (30.1) | 167 (29.5) |
| Not Hispanic or Latino | 189 (36.2) | 171 (36.5) | 373 (69.3) | 399 (70.4) |
| Not reported or unknown | 0 | 1 (<1) | 3 (<1) | 1 (<1) |
| Country, n (%) | ||||
| Russia | 0 | 0 | 136 (25.3) | 155 (27.3) |
| Ukraine | 0 | 0 | 112 (20.8) | 99 (17.5) |
| United States | 522 (100) | 468 (100) | 290 (53.9) | 313 (55.2) |
Source: Adapted from FDA Review
Microbiologic modified Intent-to-Treat population: all randomized patients who received at least a single dose of study medication and had the disease under study and a positive urine culture of uropathogen.
Abbreviations: SD, standard deviation
What are the benefits of this drug?
In Trial 1, among patients who had growth of a bacteria in their urine culture that was sensitive to the comparator antibiotic (amoxicillin/clavulanate), 61.7% of patients treated with ORLYNVAH achieved overall success compared to 55.0% of patients treated with amoxicillin clavulanate, showing the efficacy of ORLYNVAH. There were very few patients with uUTI caused by bacteria that was not sensitive to amoxicillin/clavulanate so the effect of ORLYNVAH could not be evaluated in this group of patients.
In Trial 2, among patients who had growth of a bacteria in their urine culture that was sensitive to the comparator antibiotic (ciprofloxacin), ORLYNVAH was not as effective as ciprofloxacin with 60.4% of patients treated with ORLYNVAH experiencing overall success compared to 71.8% of patients treated with ciprofloxacin. However, among patients who had growth of a bacteria in their urine culture that was not sensitive to ciprofloxacin, ORLYNVAH was effective with 48.1% of patients treated with ORLYNVAH experiencing overall success compared to 32.9% of patients treated with ciprofloxacin.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes the efficacy results at the TOC visit in the efficacy population in Trial 1 by uropathogen susceptibility to the active comparator. ORLYNVAH demonstrated efficacy in the efficacy population with uropathogens susceptible to the comparator (micro-MITTS). The efficacy population with uropathogens non-susceptible to the comparator was small (N=67) and had insufficient power to draw conclusions regarding efficacy.
Table 2. Composite Response1 at the Test-of-Cure Visit in Patients With uUTI, Efficacy Population, Trial 1
| Trial 1 Study Population |
| Amoxicillin/ |
|
|---|---|---|---|
| Efficacy population with uropathogens susceptible to the comparator (micro-MITTS) | |||
| Composite response | 296/480 (61.7) | 243/442 (55.0) | 6.7 (0.3, 13.0) |
| Clinical cure | 371/480 (77.3) | 339/442 (76.7) | 0.6 (-4.8, 6.1) |
| Microbiological response | 361/480 (75.2) | 295/442 (66.7) | 8.5 (2.6, 14.3) |
| Efficacy population with uropathogens non-susceptible to the comparator (micro-MITTR) | |||
| Composite response | 22/42 (52.4) | 17/25 (68.0) | -15.6 (-37.5, 9.1) |
| Clinical cure | 26/42 (61.9) | 18/25 (72.0) | -10.1 (-31.5, 14.0) |
| Microbiological response | 29/42 (69.0) | 20/25 (80.0) | -11.0 (-30.7, 12.0) |
Source: Adapted from ORLYNVAH Prescribing Information
1 Combined clinical and microbiological success
a 500 mg/500 mg orally twice daily for 5
b 875 mg/125 mg orally twice daily for 5 days
c The 95% CI was calculated using the unstratified Miettinen and Nurminen method
Abbreviations: CI, confidence interval; uUTI, uncomplicated urinary tract infection
Table 3 summarizes the efficacy results in the efficacy population of Trial 2.
Table 3. Efficacy Results: Composite Response1 at the Test-of-Cure Visit in Patients With uUTI, Efficacy Population, Trial 2
| Trial 2 Study Population | ORLYNVAHa | Ciprofloxacinb | Treatment Difference |
|---|---|---|---|
| Efficacy population with uropathogens susceptible to the comparator (micro-MITTS) | |||
| Composite response | 227/376 (60.4) | 300/418 (71.8) | -11.4 (-17.9, -4.8) |
| Clinical cure | 205/376 (81.1) | 351/418 (84.0) | -2.9 (-8.2, 2.4) |
| Microbiological response | 262/376 (69.7) | 336/418 (80.4) | -10.7 (-16.7, -4.7) |
| Efficacy population with uropathogens non-susceptible to the comparator (micro-MITTR) | |||
| Composite response | 78/162 (48.1) | 49/149 (32.9) | 15.3 (4.3, 25.8) 0.006 |
| Clinical cure | 136/162 (84.0) | 69/149 (46.3) | 19.5 (10.0, 29.0) |
| Microbiological response | 92/162 (56.8) | 66/149 (44.3) | 12.5 (1.4, 23.3) |
Source: Adapted from ORLYNVAH Prescribing Information
1 Combined clinical cure and microbiological response
a 500 mg/500 mg orally twice daily for 5 days
b 250 mg orally twice daily for 3 days
c The 95% CI was calculated using the unstratified Miettinen and Nurminen method
Abbreviations: CI, confidence interval; uUTI, uncomplicated urinary tract infection
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Not applicable, as the trials only enrolled female patients.
- Race: ORLYNVAH worked similarly in White and Black or African American patients. The number of patients of other races was limited; therefore, differences in how ORLYNVAH worked among other races could not be determined.
- Age: The observed effect of ORLYNVAH was similar in patients younger and older than 65 years of age.
- Ethnicity: ORLYNVAH worked similarly in Hispanic or Latino patients and those who were not Hispanic or Latino.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 4. Composite Response at the Test-of-Cure Visit in Patients With uUTI by Subgroup, Efficacy Population With Uropathogens Susceptible to Comparator (Micro-MITTS), Trial 1
| Subgroup |
| Amoxicillin/ | Treatment |
|---|---|---|---|
| Age, years | |||
| <65 | 231/367 (62.9) | 205/350 (58.6) | 4.4 (-2.8, 11.5) |
| ≥65 | 65/113 (57.5) | 38/92 (41.3) | 16.2 (2.4, 29.4) |
| Sex | |||
| Female | 296/480 (61.7) | 243/442 (55.0) | 6.7 (0.3, 13.0) |
| Race | |||
| American Indian or Alaska Native | 1/1 (100) | 0/1 (0) | NA |
| Asian | 8/10 (80.0) | 7/8 (87.5) | -7.5 (-43.2, 32.9) |
| Black or African American | 45/78 (57.7) | 39/78 (50.0) | 7.7 (-7.9, 23.3) |
| Native Hawaiian or other Pacific Islander | 0 | 0/1 (0) | NA |
| White | 235/383 (61.6) | 196/350 (56.0) | 5.6 (-1.5, 12.7) |
| Other | 6/8 (75.0) | 1/4 (25.0) | 50.0 (-10.9, 83.3) |
| Ethnicity | |||
| Hispanic or Latino | 201/304 (66.1) | 160/278 (57.6) | 8.6 (0.7, 16.4) |
| Not Hispanic or Latino | 95/176 (54.0) | 83/163 (50.9) | 3.1 (-7.6, 13.6) |
| Not reported | 0 | 0/1 (0) | NA |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; NA, not applicable; uUTI, uncomplicated urinary tract infection
Table 5. Composite Response at the Test-of-Cure Visit in Patients With uUTI by Subgroup, Efficacy Population With Uropathogens Non-Susceptible to Comparator (Micro-MITTR), Trial 2
| Subgroup | ORLYNVAH | Ciprofloxacin | Treatment Difference |
|---|---|---|---|
| Age, years | |||
| <65 | 60/104 (57.7) | 39/84 (46.4) | 11.2 (-3.1,25.2) |
| ≥65 | 18/58 (31.0) | 10/65 (15.4) | 15.6 (0.7, 30.6) |
| Sex | |||
| Female | 78/162 (48.1) | 49/149 (32.9) | 15.3 (4.3, 25.8) |
| Race | |||
| Asian | 0/2 (0) | 0 | NA |
| Black or African American | 9/15 (60.0) | 6/12 (50.0) | 10.0 (-26.7,44.7) |
| White | 69/144 (47.9) | 42/136 (30.9) | 17.0 (5.6, 28.0) |
| Other | 0/1 (0) | 1/1 (100) | NA |
| Ethnicity | |||
| Hispanic or Latino | 33/70 (47.1) | 19/59 (32.2) | 14.9 (-2.1, 31.0) |
| Not Hispanic or Latino | 45/92 (48.9) | 30/89 (33.7) | 15.2 (0.8, 29.0) |
| Not reported | 0 | 0/1 (0) | NA |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; NA, not applicable; uUTI, uncomplicated urinary tract infection
Subgroup analysis results are not reported in the efficacy population with uropathogens non-susceptible to comparator (micro-MITTR) in Trial 1 and in the efficacy population with uropathogens susceptible to comparator (micro-MITTS) in Trial 2, due to the small sample size in Trial 1 and failure to demonstrate efficacy in the micro-MITTS population in Trial 2.
What are the possible side effects?
Common side effects observed during the clinical trials of ORLYNVAH for the treatment of adult females with uUTI were diarrhea, nausea, vaginal yeast infection, headache, and vomiting.
ORLYNVAH belongs to a class of drugs that may cause serious allergic reactions or severe diarrhea caused by Clostridioides difficile.
What are the possible side effects (results of trials used to assess safety)?
The safety population includes all patients who received at least one dose of ORLYNVAH or comparator. Table 6 summarizes the most common adverse reactions observed in the trials.
Table 6. Adverse Reactions Occurring in ≥1% of Patients in the ORLYNVAH Arm, Integrated Safety Population, Trials 1 and 2
| Adverse Reaction | ORLYNVAHa | Amoxicillin/Clavulanateb | Ciprofloxacinc |
|---|---|---|---|
| Diarrhead | 194 (10) | 45 (4) | 21 (3) |
| Nausea | 80 (4) | 32 (3) | 30 (4) |
| Vulvovaginal mycotic infectione | 46 (2) | 13 (1) | 7 (1) |
| Headache | 42 (2) | 17 (2) | 18 (2) |
| Vomiting | 29 (2) | 4 (0.4) | 11 (1) |
| Abdominal painf | 22 (1) | 11 (1) | 9 (1) |
Source: ORLYNVAH Prescribing Information
a ORLYNVAH tablets (sulopenem etzadroxil 500 mg/probenecid 500 mg) one tablet twice daily for 5 days
b Amoxicillin/clavulanate tablets (875 mg/125 mg) one tablet twice daily for 5 days
c Ciprofloxacin tablets (250 mg) one tablet twice daily for 3 days.
d Diarrhea includes diarrhea and loose stools.
e Vulvovaginal mycotic infection includes vulvovaginal mycotic infection, vulvovaginal candidiasis, vaginal infection, fungal infection, genital infection fungal, and yeast infection.
f Abdominal pain includes abdominal pain, abdominal pain lower, abdominal pain upper, and abdominal discomfort.
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The trials only enrolled female participants.
- Race: The occurrence of side effects was similar in White and Black or African American patients. The number of subjects of other races was too small to draw conclusions about other racial differences in side effects.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 7 summarizes the differences in adverse events among subgroups observed in the trials.
Table 7. Adverse Events by Subgroup, Safety Population
| Subgroup |
| Amoxicillin/ |
|
|---|---|---|---|
| Sex | |||
| Female | 416/1932 (21.5) | 136/1107 (12.3) | 115/822 (14.0) |
| Age group, years | |||
| 18 to 29 | 74/336 (22.0) | 16/190 (8.4) | 21/165 (12.7) |
| 30 to 60 | 229/1002 (22.9) | 80/620 (12.9) | 47/352 (13.4) |
| ≥60 | 113/594 (19.0) | 40/297 (13.5) | 47/305 (15.4) |
| ≥65 | 90/442 (20.4) | 35/212 (16.5) | 37/233 (15.9) |
| ≥75 | 38/166 (22.9) | 15/64 (23.4) | 16/97 (16.5) |
| Race | |||
| White | 339/1601 (21.2) | 101/855 (11.8) | 104/742 (14.0) |
| American Indian or Alaska Native | 5/12 (41.7) | 0/4 (0) | 1/3 (33.3) |
| Asian | 9/30 (30.0) | 5/15 (33.3) | 1/7 (14.3) |
| Black or African American | 56/268 (20.9) | 26/217 (12.0) | 8/66 (12.1) |
| Native Hawaiian or other Pacific Islander | 0/1 (0) | 0/2 (0) | 1/1 (100) |
| Other | 7/20 (35.0) | 4/14 (28.6) | 0/3 (0) |
| Ethnicity | |||
| Hispanic or Latino | 191/1032 (18.5) | 84/760 (11.1) | 44/255 (17.3) |
| Not Hispanic or Latino | 222/895 (24.8) | 52/346 (15.0) | 71/566 (12.5) |
| Not reported | 2/4 (50.0) | 0/1 (0) | 0/1 (0) |
| Unknown | 1/1 (100) | 0/0 (NA) | 0/0 (NA) |
| Geographic region | |||
| United States | 367/1593 (23.0) | 136/1107 (12.3) | 76/461 (16.5) |
| Non-United States | 49/339 (14.5) | 0/0 (NA) | 39/361 (10.8) |
Source: Adapted from FDA Review
Abbreviations: N, number of subjects in treatment arm; n, number of subjects with adverse event; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.