Drug Trials Snapshots: QALSODY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the QALSODY Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
QALSODY (tofersen)
kal soe' dee
BIOGEN MA INC
Original Approval date: April 25, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
QALSODY is an antisense oligonucleotide (ASO) that treats adults with amyotrophic lateral sclerosis (ALS) who have a mutation in the superoxide dismutase 1 (SOD1) gene (SOD1-ALS). QALSODY is designed to reduce production of the SOD1 protein by binding to the SOD1 mRNA.
SOD1-ALS is a rare disease that causes death of the nerve cells that control voluntary muscles. Voluntary muscles produce movements such as chewing, walking, breathing, and talking. ALS causes the nerves to lose the ability to activate the muscles, leading to muscle weakness and paralysis. ALS is a progressive disease that worsens over time, and often leads to death within three to five years from when symptoms first appear.
How is this drug used?
A healthcare provider injects QALSODY directly into the cerebrospinal fluid through a needle placed in the patient’s lower back. This procedure is known as a lumbar puncture.
QALSODY is initially given as three doses administered 14 days apart. A maintenance dose is then given once every 28 days after that.
Who participated in the clinical trials?
The FDA approved QALSODY based on evidence from a single clinical study (Study 1) of 108 patients with SOD1-ALS. The trial was conducted at 32 sites in 9 countries including Belgium, Canada, Denmark, France, Germany, Italy, Japan, the United Kingdom, and the United States.
The safety of QALSODY was evaluated in 147 patients with SOD1-ALS. Safety was evaluated in the placebo-controlled Study 1 and an open-label long-term extension study (Study 2).
How were the trials designed?
The efficacy of QALSODY was evaluated in a single clinical trial of 108 patients with SOD1-ALS, who were randomly assigned to receive QALSODY or placebo for 24 weeks. Patients received QALSODY once every 14 days for three doses, and then received a dose every 28 days. Neither the patients nor the healthcare providers knew which treatment was being given. The benefit was evaluated by measuring the ALS Functional Rating Scale-Revised (ALSFRS-R) and by evaluating markers of nerve damage in the blood and spinal fluid.
How were the trials designed?
The efficacy of QALSODY was assessed in a 28-week randomized, double-blind, placebo-controlled clinical study in patients 23 to 78 years of age with weakness attributable to ALS and a SOD1 mutation confirmed by a central laboratory. A total of 108 patients were randomized 2:1 to receive treatment with either QALSODY 100 mg (N=72) or placebo (N=36) for 24 weeks (three loading doses followed by five maintenance doses). Concomitant riluzole and/or edaravone use was permitted for patients. The primary endpoint was the change from baseline to Week 28 in the ALSFRS-R total score. Secondary endpoints included change from baseline at Week 28 in plasma neurofilament light (NfL) and cerebrospinal fluid (CSF) SOD1 protein.
DEMOGRAPHICS SNAPSHOT
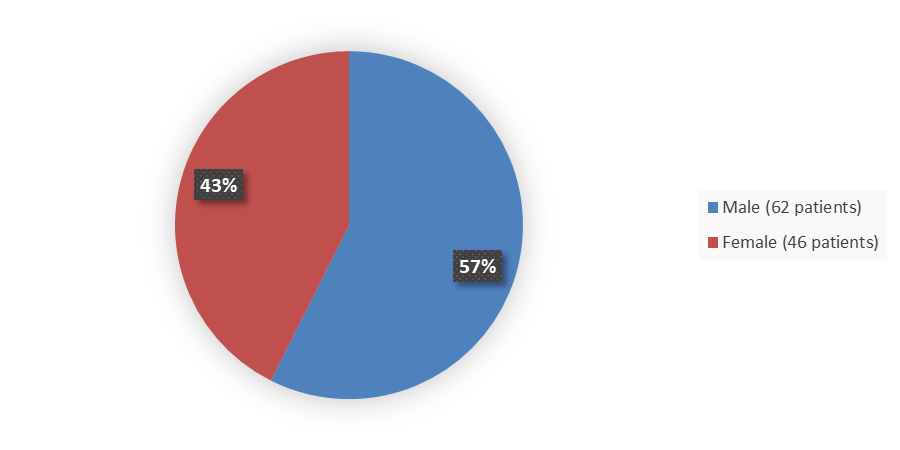
Figure 1 summarizes how many males and females were enrolled in the clinical trial used to evaluate the efficacy and safety of QALSODY.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Adapted from FDA Review
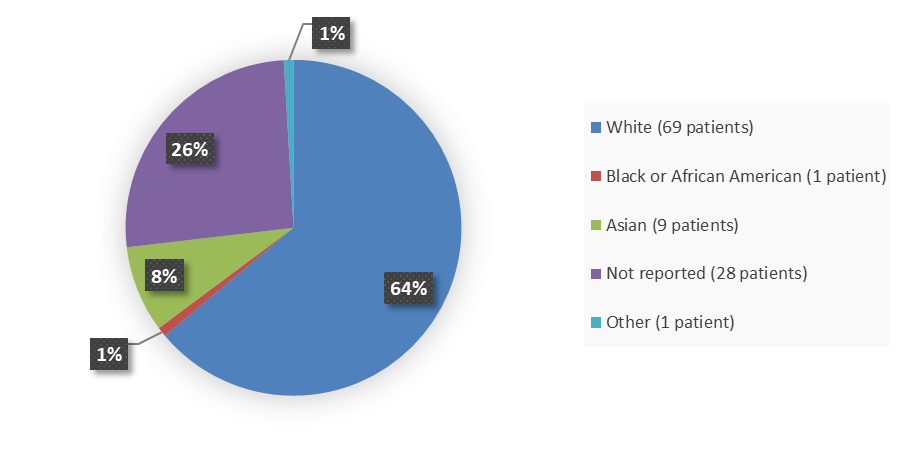
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy and safety of QALSODY.
Figure 2. Baseline Demographics by Race, Efficacy Population
Adapted from FDA Review
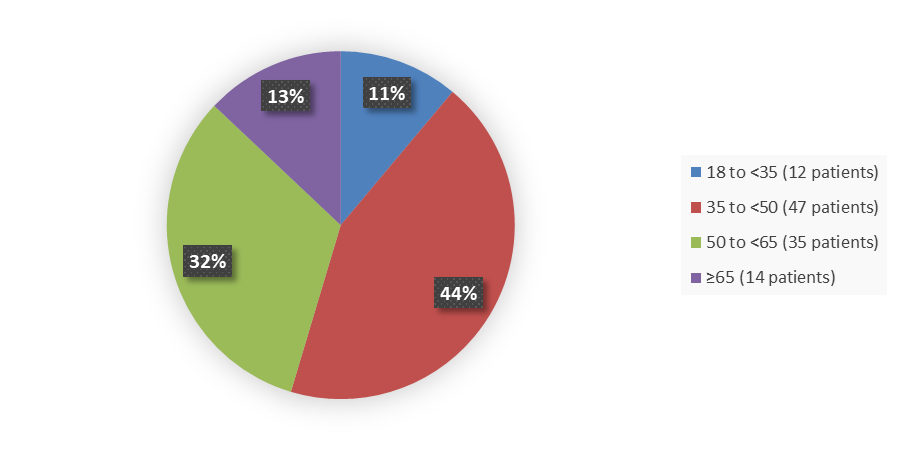
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate the efficacy and safety of QALSODY.
Figure 3. Baseline Demographics by Age, Efficacy Population
Adapted from FDA Review
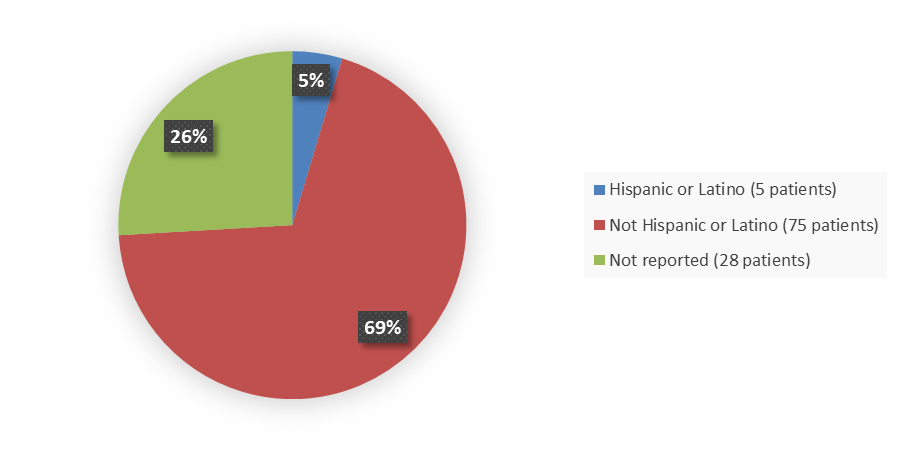
Figure 4 summarizes the percentage of patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy and safety of QALSODY.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Adapted from FDA Review
Who participated in the trials?
Demographic data for Study 1 is presented in Table 1.
Table 1. Baseline Demographic for Study 1
|
|
QALSODY |
Placebo |
|---|---|---|
|
Sex, n (%) |
||
|
Female |
29 (40.3) |
17 (47.2) |
|
Male |
43 (59.7) |
19 (52.8) |
|
Age, years |
||
|
Mean (SD) |
48.1 (12.6) |
51.2 (11.6) |
|
Median (min, max) |
47.5 (23, 78) |
51.5 (28, 73) |
|
Age group, years, n (%) |
||
|
18 to <35 |
10 (13.9) |
2 (5.6) |
|
35 to <50 |
32 (44.4) |
15 (41.7) |
|
50 to <65 |
21 (29.2) |
14 (38.9) |
|
≥65 |
9 (12.5) |
5 (13.9) |
|
Age group ≥75, years, n (%) |
||
|
≥75 |
1 (1.4) |
0 |
|
Race, n (%) |
||
|
White |
44 (61.1) |
25 (69.4) |
|
Asian |
5 (6.9) |
4 (11.1) |
|
Black or African American |
1 (1.4) |
0 |
|
Not reported |
21 (29.2) |
7 (19.4) |
|
Other |
1 (1.4) |
0 |
|
Ethnicity, n (%) |
||
|
Hispanic or Latino |
4 (5.6) |
1 (2.8) |
|
Not Hispanic or Latino |
47 (65.3) |
28 (77.8) |
|
Not reported |
21 (29.2) |
7 (19.4) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
Patients receiving QALSODY had a bigger reduction in NfL, a marker of nerve cell death found in the blood, compared to patients receiving placebo. The findings are reasonably likely to predict a clinical benefit in these patients.
QALSODY was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
This indication is approved under accelerated approval based on reduction in plasma NfL, a biomarker of nerve injury and neurodegeneration. There was a nominally significant greater reduction in NfL observed in patients treated with QALSODY compared to patients receiving placebo. The findings are reasonably likely to predict a clinical benefit in patients. Continued approval for this indication may depend on verification of clinical benefit in confirmatory trials.
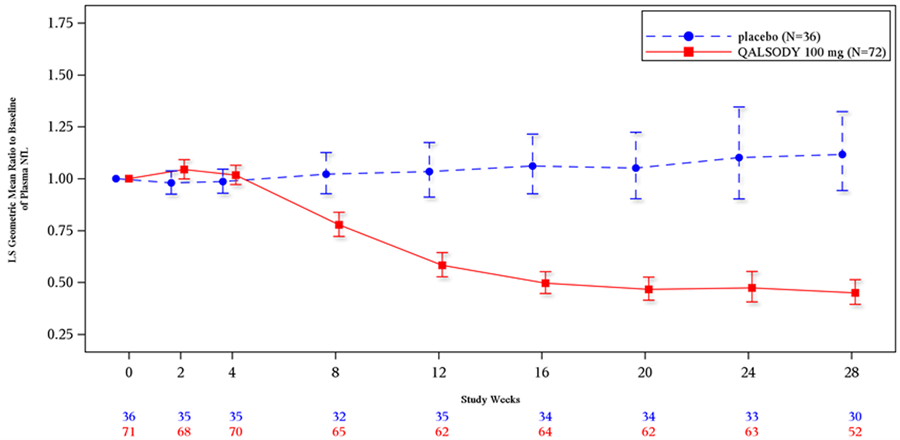
Figure 5. Plasma NfL Adjusted Geometric Mean Ratio to Baseline Values in Study 1 Part C by Study Week for the Efficacy Population
Source: QALSODY Prescribing Information
Abbreviations: LS, least squares; NfL, neurofilament light
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: QALSODY led to similar reductions in plasma NfL in males and females.
- Race: The majority of patients in the trial were White. Differences in how well QALSODY worked among races could not be determined because of the small number of patients in other races.
- Age: The clinical studies did not assess the effect of age on QALSODY’s ability to reduce plasma NfL in patients.
What are the possible side effects?
The most common side effects (≥10% of patients treated with QALSODY and greater than placebo) were pain, fatigue, arthralgia (joint pain), increased cerebrospinal (brain and spinal cord) fluid white blood cells, and myalgia (muscle pain).
QALSODY may cause serious neurologic side effects of myelitis and/or radiculitis (inflammation of spinal cord and nerve roots), papilledema (swelling of optic nerve in the eyes) and elevated intracranial pressure, and aseptic meningitis (inflammation of the fluid around the brain and spinal cord).
What are the possible side effects (results of trials used to assess safety)?
Table 2 below summarizes the most common adverse events that occurred in Study 1.
Table 2. Adverse Drug Reactions That Occurred in At Least 5% of Patients Treated with QALSODY and at >5% Higher Frequency Than Placebo, Study 1
|
Adverse Reaction |
Study 1 Part C |
|
|---|---|---|
|
QALSODY |
Placebo |
|
|
Pain1 |
42 |
22 |
|
Fatigue |
17 |
6 |
|
Arthralgia |
14 |
6 |
|
CSF white blood cell increased2 |
14 |
0 |
|
Myalgia |
14 |
6 |
|
CSF protein increased |
8 |
3 |
|
Musculoskeletal stiffness |
6 |
0 |
|
Neuralgia |
6 |
0 |
Source: QALSODY Prescribing Information
1 Pain includes preferred terms of pain, back pain, and pain in extremity
2 CSF white blood cell increased includes preferred terms of CSF white blood cell increased and pleocytosis
Abbreviations: CSF, cerebrospinal fluid
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients in the trial were White. Differences in side effects among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 3. Overview of Adverse Events by Demographic Subgroup, Study 1
|
Demographic |
QALSODY |
Placebo |
|---|---|---|
|
Sex |
||
|
Female |
28/29 (96.6) |
17/17 (100) |
|
Male |
41/43 (95.3) |
17/19 (89.5) |
|
Age group, years |
||
|
18 to <35 |
10/10 (100) |
2/2 (100) |
|
35 to <50 |
30/32 (93.8) |
15/15 (100) |
|
50 to <65 |
20/21 (95.2) |
14/14 (100) |
|
≥65 |
9/9 (100) |
3/5 (60.0) |
|
Age group ≥75, years |
||
|
≥75 |
1/1 (100) |
0/0 (NA) |
|
Race |
||
|
White |
42/44 (95.5) |
23/25 (92.0) |
|
Asian |
5/5 (100) |
4/4 (100) |
|
Black or African American |
1/1 (100) |
0/0 (NA) |
|
Not reported |
20/21 (95.2) |
7/7 (100) |
|
Other |
1/1 (100) |
0/0 (NA) |
|
Ethnicity |
||
|
Hispanic or Latino |
4/4 (100) |
1/1 (100) |
|
Not Hispanic or Latino |
45/47 (95.7) |
26/28 (92.0) |
|
Not reported |
20/21 (95.2) |
7/7 (100) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients meeting criteria; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.