Drug Trials Snapshots: REZUROCK
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when considering the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the REZUROCK Prescribing Information for all of the approved conditions of use for this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
REZUROCK (belumosudi)
(Rez'-ur-ok )
Kadmon Pharmaceuticals LLC
Approval date: July 16, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
REZUROCK is a drug used in adults and pediatric patients 12 years and older with chronic graft-versus-host disease (chronic GVHD) after failure of at least two prior treatments.
Chronic GVHD is a complication that can occur after stem cell or bone marrow transplantation in which the transplanted donor cells attack the transplant recipient’s body.
How is this drug used?
REZUROCK is a 200 mg tablet taken by mouth once daily with food.
Who participated in the clinical trials?
The FDA approved REZUROCK based on evidence from one clinical trial of 65 patients with chronic GVHD to evaluate efficacy of REZUROCK at 200 mg once daily. A total of eighty-three (83) patients were evaluated for safety; therefore, the number of patients representing efficacy findings may differ from the number of patients representing safety findings due to different pools of study participants analyzed for efficacy and safety. The trial was conducted at 28 sites in the United States.
How were the trials designed?
REZUROCK was evaluated in one single-arm clinical trial of 65 patients with chronic GVHD for efficacy. A total of eighty-three (83) patients were evaluated for safety. All patients received 200 mg REZUROCK once daily until chronic GHVD worsens that requires new treatment.
The benefit of REZUROCK was evaluated by measuring how many patients had responded to the treatment and by how long that response lasted.
How were the trials designed?
There were two single-arm, open-label, multicenter studies of REZUROCK in patients with chronic GVHD who had received prior treatments. Sixty-five (65) patients who had received at least 2 prior treatments were treated with 200 mg REZUROCK once daily to evaluate efficacy in one study. Eighty-three (83) patients were treated with 200 mg REZUROCK once daily in the studies to evaluate safety.
The efficacy outcome measures were overall response rate (ORR), according to the 2014 NIH consensus criteria, and duration of response (DOR).
What are the benefits of this drug?
Seventy-five percent of 65 patients who were treated with REZUROCK experienced complete or partial response. The time from treatment to first response was 1.8 months. The majority of responding patients (62 percent) did not need additional treatment for at least 12 months after response.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results based on overall response rate (ORR) through Cycle 7 Day 1 according to the 2014 NIH Response Criteria.
Table 1. Efficacy Results of Pivotal Clinical Trial
| Efficacy Endpoints | Results (N=65) |
|---|---|
| Overall Response Rate (ORR) Overall response rate, (95% CI) Complete Response Partial Response | 49 (75%) (63%, 85%) 4 (6%) 45 (69%) |
| Duration of Response (DOR) Median duration of response, (95% CI) | 1.9 months (1.2 to 2.9 months) |
CI: Confidence interval
NIH: National Institutes of Health
Source: REZUROCK Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: REZUROCK worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how REZUROCK worked among races could not be determined.
- Age: The number of patients over 65 years of age was small; therefore, differences in how REZUROCK worked among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes the efficacy results of overall response rate (ORR) by sex, age, and race.
Table 2. Overall Response Rate (ORR) through Cycle 7 Day 1 by Subgroup in Patients Treated with REZUROCK
| ORR (Responders/N) | 95% CI* | |
| Sex | ||
| Male | 76% (32/42) | 61, 88 |
| Female | 74% (17/23) | 52, 90 |
| Age Group | ||
| <65 years | 83% (40/48) | 70, 93 |
| ≥65 years | 53% (9/17) | 28, 77 |
| Race | ||
| White | 74% (40/54) | 60, 85 |
| Black or African-American | 100% (6/6) | 54, 100 |
| Asian Indian | 0% (0/1) | 0, 98 |
| Unknown | 75% (3/4) | 19, 99 |
*Estimated 95% Confidence Interval using the Clopper-Pearson method
What are the possible side effects?
The most common side effects of REZUROCK are infection, tiredness or weakness, nausea, diarrhea, shortness of breath, cough, swelling, bleeding, stomach (abdominal) pain, muscle or bone pain, headache, and high blood pressure.
Based on animal studies, REZUROCK can be harmful to an unborn baby when given to a pregnant woman.
What are the possible side effects (results of trials used to assess safety)?
Table 3 and Table 4 below summarize adverse reactions (side effects) and electrolyte abnormalities in the clinical trial, respectively.
Table 3. Adverse Reactions in > 10% Patients with Chronic GVHD Treated with REZUROCK
| Adverse Reaction | REZUROCK 200 mg once daily (N=83) | |
|---|---|---|
| All Grades (%) | Grades 3-4 (%) | |
| Infections and infestations | ||
| General disorders and administration site conditions | ||
| Respiratory, thoracic and mediastinal | ||
| Musculoskeletal and connective tissue | ||
| Nervous system | ||
| Metabolism and nutrition | ||
| Skin and subcutaneous | ||
| Infection (pathogen not specified)a | 53 | 16 |
| Viral infectionb | 19 | 4 |
| Bacterial infectionc | 16 | 4 |
| Astheniad | 46 | 4 |
| Edemae | 27 | 1 |
| Pyrexia | 18 | 1 |
| Gastrointestinal | ||
| Nauseaf | 42 | 4 |
| Diarrhea | 35 | 5 |
| Abdominal paing | 22 | 1 |
| Dysphagia | 16 | 0 |
| Dyspneah | 33 | 5 |
| Coughi | 30 | 0 |
| Nasal congestion | 12 | 0 |
| Vascular | ||
| Hemorrhagej | 23 | 5 |
| Hypertension | 21 | 7 |
| Musculoskeletal paink | 22 | 4 |
| Muscle spasm | 17 | 0 |
| Arthralgia | 15 | 2 |
| Headachel | 21 | 0 |
| Decreased appetite | 17 | 1 |
| Rashm | 12 | 0 |
| Pruritusn | 11 | 0 |
a infection with an unspecified pathogen includes acute sinusitis, device related infection, ear infection, folliculitis, gastroenteritis, gastrointestinal infection, hordeolum, infectious colitis, lung infection, skin infection, tooth infection, urinary tract infection, wound infection, upper respiratory tract infection, pneumonia, conjunctivitis, sinusitis, respiratory tract infection, bronchitis, sepsis, septic shock.
b includes influenza, rhinovirus infection, gastroenteritis viral, viral upper respiratory tract infection, bronchitis viral, Epstein-Barr viremia, Epstein-Barr virus infection, parainfluenzae virus infection, Varicella zoster virus infection, viral infection.
c includes cellulitis, Helicobacter infection, Staphylococcal bacteremia, catheter site cellulitis, Clostridium difficile colitis, Escherichia urinary tract infection, gastroenteritis Escherichia coli, Pseudomonas infection, urinary tract infection bacterial.
d includes fatigue, asthenia, malaise.
e includes edema peripheral, generalized edema, face edema, localized edema, edema.
f includes nausea, vomiting.
g includes abdominal pain, abdominal pain upper, abdominal pain lower.
h includes dyspnea, dyspnea exertional, apnea, orthopnea, sleep apnea syndrome.
i includes cough, productive cough.
j includes contusion, hematoma, epistaxis, increased tendency to bruise, conjunctival hemorrhage, hematochezia, mouth hemorrhage, catheter site hemorrhage, hematuria, hemothorax, purpura.
k includes pain in extremity, back pain, flank pain, limb discomfort, musculoskeletal chest pain, neck pain, musculoskeletal pain.
l includes headache, migraine.
m includes rash, rash maculo-papular, rash erythematous, rash generalized, dermatitis exfoliative.
n includes pruritus, pruritus generalized.
Source: REZUROCK Prescribing Information
Table 4. Selected Laboratory Abnormalities in Patients with Chronic GVHD Treated with REZUROCK
| REZUROCK 200 mg once daily | |||
|---|---|---|---|
| Grade 0‐1 Baseline | Grade 2‐4 Max Post | Grade 3‐4 Max Post | |
| Parameter | (N) | (%) | (%) |
| Chemistry | |||
| Phosphate Decreased | 76 | 28 | 7 |
| Gamma Glutamyl Transferase Increased | 47 | 21 | 11 |
| Calcium Decreased | 82 | 12 | 1 |
| Alkaline Phosphatase Increased | 80 | 9 | 0 |
| Potassium Increased | 82 | 7 | 1 |
| Alanine Aminotransferase Increased | 83 | 7 | 2 |
| Creatinine Increased | 83 | 4 | 0 |
| Hematology | |||
| Lymphocytes Decreased | 62 | 29 | 13 |
| Hemoglobin Decreased | 79 | 11 | 1 |
| Platelets Decreased | 82 | 10 | 5 |
| Neutrophil Count Decreased | 83 | 8 | 4 |
Source: REZUROCK Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar in White and Black or African American patients.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize side effects during the clinical trial by age, sex, and age subgroup.
Table 5. Treatment Emergent Adverse Events by Age
| < 65 years old (N=66) | > 65 years old (N=17) | |||
| Grouped Terms | n | (%) | n | (%) |
| Tachycardia | 3 | 5 | 4 | 24 |
| Decreased appetite | 9 | 14 | 5 | 29 |
| Haemorrhage | 13 | 20 | 6 | 35 |
| Insomnia | 3 | 5 | 3 | 18 |
| Laceration | 0 | 0 | 2 | 12 |
| Abdominal pain | 13 | 20 | 5 | 29 |
| Chills | 7 | 11 | 0 | 0 |
| Stomatitis | 7 | 11 | 0 | 0 |
| Arthralgia | 11 | 17 | 1 | 6 |
| Headache | 15 | 23 | 2 | 12 |
| Pruritus | 8 | 12 | 0 | 0 |
| Musculoskeletal pain | 16 | 24 | 2 | 12 |
| Muscle spasms | 13 | 20 | 1 | 6 |
| Nasal congestion | 10 | 15 | 0 | 0 |
| Nausea | 30 | 45 | 5 | 29 |
| Asthenia | 33 | 50 | 5 | 29 |
Source: Adapted from FDA Safety Review
Table 6. Treatment Emergent Adverse Events by Sex
| Grouped Terms | n | (%) | n | (%) |
|---|---|---|---|---|
| Females (N=28) | Males (N=55) | |||
| Abdominal pain | 3 | 11 | 15 | 27 |
| Muscular weakness | 0 | 0 | 8 | 15 |
| Musculoskeletal pain | 4 | 14 | 14 | 25 |
| Renal failure | 1 | 4 | 8 | 15 |
| Nausea | 10 | 36 | 25 | 45 |
| Pruritus | 5 | 18 | 4 | 7 |
| Infection | 17 | 61 | 27 | 49 |
| Diarrhoea | 12 | 43 | 17 | 31 |
| Dry mouth | 5 | 18 | 3 | 5 |
| Bacterial infection | 7 | 25 | 6 | 11 |
| Oedema | 11 | 39 | 11 | 20 |
| Dyspnoea | 13 | 46 | 14 | 25 |
Source: Adapted from FDA Safety Review
Table 7. Treatment Emergent Adverse Events by Race
| White (N=70) | Not White (N=13) | |||
|---|---|---|---|---|
| Grouped Terms | n | (%) | n | (%) |
| Decreased appetite | 8 | 11 | 6 | 46 |
| Dyspnoea | 20 | 29 | 7 | 54 |
| Nausea | 27 | 39 | 8 | 62 |
| Blood creatine phosphokinase increased | 1 | 1 | 3 | 23 |
| Pain | 1 | 1 | 3 | 23 |
| Headache | 12 | 17 | 5 | 38 |
| Abdominal pain | 13 | 19 | 5 | 38 |
| Cough | 19 | 27 | 6 | 46 |
| Dysphagia | 9 | 13 | 4 | 31 |
| Arthropod bite | 0 | 0 | 2 | 15 |
| Eye swelling | 0 | 0 | 2 | 15 |
| Pruritus | 6 | 9 | 3 | 23 |
| Pollakiuria | 1 | 1 | 2 | 15 |
| Diarrhoea | 23 | 33 | 6 | 46 |
| Hypomagnesaemia | 2 | 3 | 2 | 15 |
| Toothache | 2 | 3 | 2 | 15 |
| Hypophosphataemia | 3 | 4 | 2 | 15 |
| Musculoskeletal pain | 14 | 20 | 4 | 31 |
| Myalgia | 4 | 6 | 2 | 15 |
| Weight decreased | 4 | 6 | 2 | 15 |
| Asthenia | 31 | 44 | 7 | 54 |
| Tachycardia | 7 | 10 | 0 | 0 |
| Liver function test abnormal | 18 | 26 | 2 | 15 |
| Muscle spasms | 13 | 19 | 1 | 8 |
| Dry mouth | 8 | 11 | 0 | 0 |
| Oedema | 20 | 29 | 2 | 15 |
| Viral infection | 15 | 21 | 1 | 8 |
| Hypertension | 16 | 23 | 1 | 8 |
| Infection | 42 | 60 | 2 | 15 |
Source: Adapted from FDA Safety Review
Demographic Snapshot
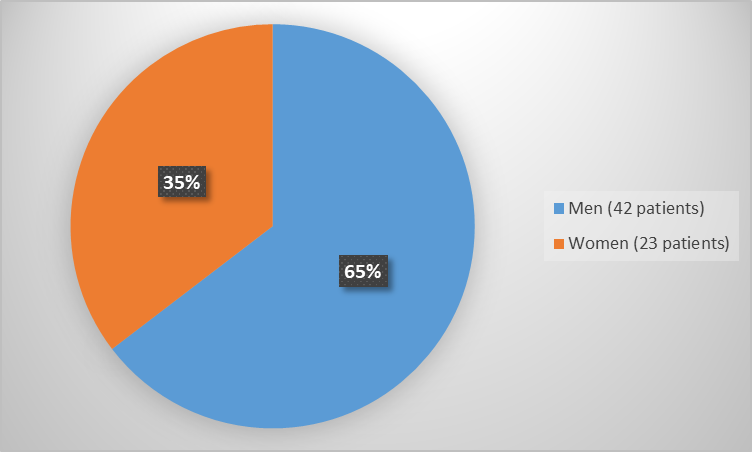
Figure 1 summarizes how many males and females were enrolled in the clinical trial used to evaluate the efficacy of REZUROCK.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: REZUROCK Prescribing Information
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of REZUROCK.
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: REZUROCK Prescribing Information
Table 8. Demographics of Efficacy Trial by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 54 | 83 |
| Black | 6 | 9 |
| Other or not reported | 5 | 8 |
Source: REZUROCK Prescribing Information
Figure 3 summarizes how many patients of certain age were enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: REZUROCK Prescribing Information
Who participated in the trials?
Table 9. Baseline Demographics of Patients in the Pivotal Clinical Trial
| REZUROCK 200 mg once daily (N=65) | |
|---|---|
| Age, Median, Years (minimum, maximum) | 53 (21, 77) |
| Age ≥ 65 Years, n (%) | 17 (26) |
| Male, n (%) | 42 (65) |
| Race, n (%) | |
| White | 54 (83) |
| Black | 6 (9) |
| Other or Not Reported | 5 (8) |
| Median (range) time (months) from Chronic GVHD Diagnosis | 25.3 (1.9, 162.4) |
| ≥ 4 Organs Involved, n (%) | 31 (48) |
| Median (range) Number of Prior Lines of Therapy | 3 (2, 6) |
| Number of Prior Lines of Therapy, n (%) | |
| 2 | 23 (35) |
| 3 | 12 (19) |
| 4 | 15 (23) |
| ≥ 5 | 15 (23) |
| Prior chronic GVHD treatment with ibrutinib, n (%) | 21 (32) |
| Prior chronic GVHD treatment with ruxolitinib, n (%) | 20 (31) |
| Refractory to Last Therapy, n (%a) | 43/55 (78) |
| Severe chronic GVHD, n (%) | 46 (71) |
| Median (range) Global Severity Rating | 7 (2, 9) |
| Median (range) Lee Symptom Scale Score at baseline | 27 (7, 56) |
| Median (range) Corticosteroid dose at baseline (PE/kg)b | 0.19 (0.03, 0.95) |
a Denominator excludes patients with unknown status
b Prednisone equivalents/kilogram
Source: REZUROCK Prescribing Information
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.