Drug Trials Snapshots: Tecentriq
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to TECENTRIQ Prescribing Information for complete information.
TECENTRIQ (atezolizumab)
(te sen' trik)
Genentech Inc.
Approval date: May 18, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TECENTRIQ is a drug for the treatment of a type of bladder cancer called urothelial carcinoma. TECENTRIQ may be used when:
- bladder cancer has spread or cannot be removed by surgery (advanced urothelial carcinoma) and,
- chemotherapy that contains platinum did not work or is no longer working.
How is this drug used?
TECENTRIQ is given by a health care professional into vein through an intravenous (IV) line over 30 to 60 minutes.
TECENTRIQ is usually given every 3 weeks.
What are the benefits of this drug?
About 15% of participants had partial or complete shrinkage of their tumors. Most of these responding participants are being followed to see how long the benefit will last.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the clinical trial based on objective response rate (ORR) as assessed by independent review facility (IRF) using Response Evaluation Criteria in Solid Tumors (RECIST v1.1) and duration of response (DoR).
Table 2. Summary of Efficacy
| All Patients | PD-L1 Expression Subgroups | ||
|---|---|---|---|
| N = 310 | P-L1 Expression of 5%="" in="">1 (N=210) | PD-L1 Expression of ≥ 5% in ICs1 (N=100) | |
| Number of IRF-assessed Confirmed Responders | 46 | 20 | 26 |
| ORR % (95% CI) | 14.8% (11.1, 19.3) | 9.5% (5.9, 14.3) | 26.0% (17.7, 35.7) |
| Complete Response (CR) (%) | 5.5% | 2.4% | 12.0% |
| Partial Response (PR) (%) | 9.4% | 7.1% | 14.0% |
| Median DoR, months (range) | NR (2.1+, 13.8+) | 12.7 (2.1+, 12.7) | NR (4.2, 13.8+) |
| NR= Not reached + Denotes a censored value 1PD-L1 expression in tumor-infiltrating immune cells (ICs) | |||
TECENTRIQ Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: TECENTRIQ worked similarly in men and women
- Race: The majority of participants in the clinical trials were White. Differences among races could not be determined due to small number of participants from other races.
- Age: TECENTRIQ worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by subgroup
Table 3. Subgroup Analysis Based on Objective Response Rate (ORR)
| ORR n/N % (95% CI) | TECENTRIQ (N=310) |
|---|---|
| Sex | |
| Men | 40/241 16.6% (12.1%, 21.9%) |
| Women | 6/69 8.7% (3.3%, 18.0%) |
| Age | |
| Age > | 17/127 13.4% (8.0%, 20.6%) |
| Age >=65 | 29/183 15.8% (10.9%, 22.0%) |
| Race | |
| White | 40/282 14.2% (10.3,18.8) |
| Non-White | 6/28 21.4 % (8.3, 41.0) |
| Region | |
| US | 28/210 13.3% (9.1%, 18.7%) |
| Non-US | 18/100 18.0% (11.0%, 27.0%) |
FDA Review
What are the possible side effects?
The most common side effects are feeling tired (fatigue), decreased appetite, nausea, urinary tract infection, fever, and constipation.
TECENTRIQ may cause serious side effects including infection, lung inflammation (pneumonitis), liver (hepatitis), intestine (colitis), and hormone glands injury (pituitary, thyroid, adrenal, and pancreas).
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in patients treated with TECENTRIQ.
Table 4. All Grade Adverse Reactions in ≥ 10% of Patients with Urothelial Carcinom
| TECENTRIQ N = 310 | ||
|---|---|---|
| Adverse Reaction | All Grades (%) | Grades 3 – 4 (%) |
| All Adverse Reactions | 96 | 50 |
| Gastrointestinal Disorders | ||
| Nausea | 25 | 2 |
| Constipation | 21 | 0.3 |
| Diarrhea | 18 | 1 |
| Abdominal pain | 17 | 4 |
| Vomiting | 17 | 1 |
| General Disorders and Administration | ||
| Fatigue | 52 | 6 |
| Pyrexia | 21 | 1 |
| Peripheral edema | 18 | 1 |
| Infections and Infestations | ||
| Urinary tract infection | 22 | 9 |
| Metabolism and Nutrition Disorders | ||
| Decreased appetite | 26 | 1 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Back/Neck pain | 15 | 2 |
| Arthralgia | 14 | 1 |
| Renal and urinary disorders | ||
| Hematuria | 14 | 3 |
| Respiratory, Thoracic, and Mediastinal Disorders | ||
| Dyspnea | 16 | 4 |
| Cough | 14 | 0.3 |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 15 | 0.3 |
| Pruritus | 13 | 0.3 |
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of common side effects was similar in male and female patients.
- Race: The majority of participants in the clinical trial were White. Differences in side effects among races could not be determined due to small number of participants from other races.
- Age: The occurrence of common side effect was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse events by subgroup
Table 5. Grade 1-4 Adverse Events by Subgroup
| Demographic Parameters | TECENTRIQ (N=310) |
|---|---|
| Percent of the Patients in the Subgroup Experiencing at Least One Adverse Event of Any Grade | |
| Sex (N) | |
| Men (241) | 98% |
| Women (69) | 91% |
| Age Group (N) | |
| 65 years=""> | 96% |
| 65 and older (183) | 95% |
| Race (N) | |
| White (282) | 96% |
| Non-White (28) | 96% |
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TECENTRIQ based primarily on evidence from one clinical trial of 310 patients with advanced bladder cancer. The trial was conducted in the United States, Canada, Spain, France, Great Britain, Germany, Italy and Netherlands.
The figure below summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
Clinical trial data
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
| White | 282 | 91% |
| Asian | 7 | 2% |
| Black or African American | 6 | 2% |
| American Indian or Alaska Native | 1 | less than 1% |
| Native Hawaiian or Other Pacific Islander | 1 | less than 1% |
| Other | 7 | 2% |
| Unknown | 6 | 2% |
Clinical trial data
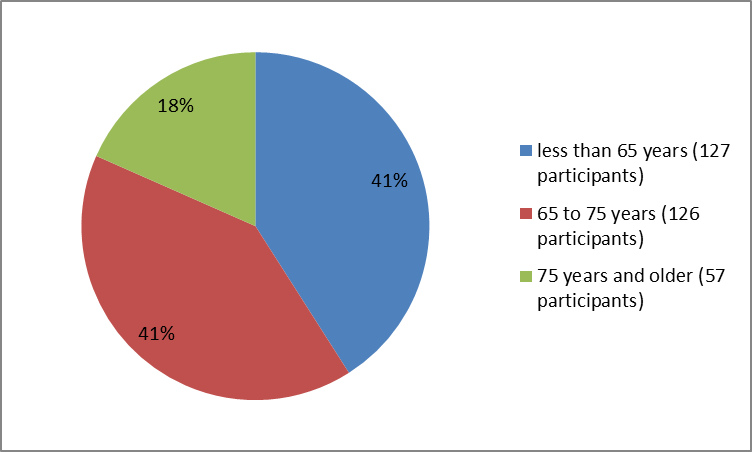
Figure 3 summarizes the percentage of patients by age group in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 6. Baseline Demographics of Patients in the Clinical Trial (Safety Population)
| Demographic Parameters | TECENTRIQ (N=310) n (%) |
|---|---|
| Sex | |
| Men | 241 (78) |
| Women | 69 (22) |
| Age | |
| Mean years (SD) | 65.6 (10.1) |
| Median (years) | 66 |
| Min, max (years) | 32,91 |
| Age Group | |
| 65=""> | 127 (41) |
| 65-75 years | 126 (41) |
| ≥75 years | 57 (18) |
| Race | |
| White | 282 (91) |
| Black or African American | 6 (2) |
| Asian | 7 (2) |
| American Indian or Alaska Native | 1 (> |
| Native Hawaiian or Other Pacific Islander | 1 (> |
| Other | 7 (2) |
| Unknown | 6 (2) |
| Ethnicity | |
| Hispanic or Latino | 10 (3) |
| Not Hispanic or Latino | 284 (92) |
| Not reported | 9 (3) |
| Unknown | 7 (2) |
| Region | |
| US | 210 (68) |
| Non-US | 100 (32) |
FDA Review
How were the trials designed?
There was one trial that evaluated benefit and side effects of TECENTRIQ. All patients had urothelial cancer that has spread and all received TELECENTRIQ every 3 weeks until either unacceptable side effects or disease progression.
The benefit of TECENTRIQ was evaluated by measuring the percentage of patients who experienced complete or partial shrinkage of their tumors (called objective response rate) and how long the benefit lasts.
How were the trials designed?
The safety and efficacy of TECENTRIQ was evaluated in a single, multicenter, open-label trial that included patients with locally advanced or metastatic urothelial carcinoma.
Patients received an intravenous infusion of TECENTRIQ every 3 weeks until unacceptable toxicity or either radiographic or clinical progression.
Primary efficacy was evaluated confirmed objective response rate (ORR) as assessed by independent review facility using Response Evaluation Criteria in Solid Tumors (RECIST v1.1) and duration of response (DoR).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.