Drug Trials Snapshots: VARUBI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the VARUBI Prescribing Information for complete information.
VARUBI (rolapitant)
(vuh ROO bee)
Tesaro Inc.
Approval date: September 1, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VARUBI is a drug used to prevent a delayed form of nausea and vomiting that can occur more than 24 hours after cancer treatment (chemotherapy).
How is this drug used?
VARUBI is a tablet that is taken one to two hours before chemotherapy. It is taken along with other medicines used to prevent nausea and vomiting.
VARUBI should be taken on the first day of each cycle of chemotherapy and not more than one time every 14 days.
What are the benefits of this drug?
Patients taking VARUBI experienced less vomiting and less need for additional medication to treat nausea or vomiting than patients receiving a “sugar pill” (placebo).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the results of the three trials. This was based on the primary endpoint of “complete response”, defined as no vomiting and no use of additional medication for symptoms of nausea or vomiting, during the delayed phase (25 to 120 hours after the start of the first cycle of chemotherapy). Granisetron and dexamethasone were also given to both the and the placebo treatment group.
Table 2. Efficacy Results Based on Complete Response Endpoint
| HEC1 Trial 1 | HEC1 Trial 2 | MEC2 Trial | ||||||
|---|---|---|---|---|---|---|---|---|
| (N=264) Rate | Control (N=262) Rate | P‑Value Treatment Difference, (95% C.I.) | (N=271) Rate | Control (N=273) Rate | P‑Value Treatment Difference, (95% C.I.) | (N=666) Rate | Control (N=666) Rate | P‑Value Treatment Difference (95% C.I.) |
| 72.7% | 58.4% | <0.001*> 14.3 ( 6.3,22.4) | 70.1% | 61.9% | 0.043* 8.2 ( 0.3, 16.1) | 71.3% | 61.6% | <0.001*> 9.8 ( 4.7, 14.8) |
1 Highly Emetogenic Chemotherapy
2 Moderately Emetogenic Chemotherapy
* Results were obtained based on the Cochran-Mantel-Haenszel test stratified by sex.
Source: Prescribing Information, Section 14, Table 7
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: VARUBI worked similarly in men and women.
- Race: VARUBI worked similarly in all races studied.
- Age: VARUBI worked similarly in patients younger than 65 years and patients 65 years and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarizes the response to by subgroups for the two HEC (highly emetogenic chemotherapy) trials, referred to as HEC Trial #1 and HEC Trial #2, and the one MEC (moderately emetogenic chemotherapy) trial.
Table 3. Efficacy comparison of Sex assessed by the complete response in the delayed phase using the Modifed Intent To Treat population—HEC Trial #1
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Controla p-value | |
|---|---|---|---|---|
| Females | ||||
| Males | ||||
| Control | 112 | 60 (53.6) | 15.5 | 0.018* |
| Rolapitant | 110 | 76 (69.1) | ||
| Control | 150 | 93 (62) | 13.3 | 0.012* |
| Rolapitant | 154 | 116 (75.3) | ||
From FDA Statistical Review
a: Analysis via Cochran Mantel-Haenszel test stratified by sex.
*: Significant at two-sided significance level of 0.05
Table 4. Efficacy comparison of Race groups assessed by the complete response in the delayed phase using the Modifed Intent To Treat population—HEC Trial #1
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Controla p-value | |
|---|---|---|---|---|
| Whites | ||||
| Non-whites | ||||
| Control | 179 | 117 (65.4) | 12.1 | 0.011* |
| Rolapitant | 178 | 138 (77.5) | ||
| Control | 83 | 36 (43.4) | 19.4 | 0.011* |
| Rolapitant | 86 | 54 (62.8) | ||
From FDA Statistical Review
a: Analysis via Cochran Mantel-Haenszel test stratified by sex.
*: Significant at two-sided significance level of 0.05
Table 5. Efficacy comparison of Age groups assessed by the complete response in the delayed phase using the Modifed Intent To Treat population—HEC Trial #1
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Controla p-value | |
|---|---|---|---|---|
| Age <> | ||||
| Age ≥65 | ||||
| Control | 193 | 117 (61) | 12 | 0.01* |
| Rolapitant | 199 | 145 (73) | ||
| Control | 69 | 36 (52) | 20 | 0.02* |
| Rolapitant | 65 | 47 (72) | ||
From FDA Statistical Review
a: Analysis via Cochran Mantel-Haenszel test stratified by sex.
*: Significant at two-sided significance level of 0.05
Table 6. Efficacy comparison of sex assessed by the complete response in the delayed phase using the Modifed Intent To Treat population--HEC Trial #2
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Controla p-value | |
|---|---|---|---|---|
| Women | ||||
| Men | ||||
| Control | 87 | 44 (50.6) | 23.3 | 0.0015* |
| Rolapitant | 89 | 65 (73.9) | ||
| Control | 186 | 125 (67.2) | 1.1 | 0.821 |
| Rolapitant | 183 | 125 (68.3) | ||
From FDA Statistical Review
a: Analysis via Cochran Mantel-Haenszel test stratified by sex.
*: Significant at two-sided significance level of 0.05
Table 7. Efficacy comparison of Race groups assessed by the complete response in the delayed phase using the Modifed Intent To Treat population--HEC Trial #2
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Control p-value | |
|---|---|---|---|---|
| Whites | ||||
| Non-whites | ||||
| Control | 212 | 135 (63.7) | 6.7 | 0.138 |
| Rolapitant | 226 | 159 (70.4) | ||
| Control | 61 | 34 (55.7) | 13.2 | 0.171 |
| Rolapitant | 45 | 31 (68.9) | ||
FDA Statistical Review
Table 8. Efficacy comparison of Age groups assessed by the complete response in the delayed phase using the Modifed Intent To Treat population--HEC Trial #2
| Age Group | n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Controla p-value |
|---|---|---|---|---|
| Age <> | ||||
| Age ≥65 | ||||
| Control | 200 | 118 (59) | 11 | 0.026* |
| Rolapitant | 198 | 138 (70) | ||
| Control | 73 | 51 (70) | 1 | 0.86 |
| Rolapitant | 73 | 52 (71) | ||
FDA Statistical Review
a: Analysis via Cochran Mantel-Haenszel test stratified by sex.
*: Significant at two-sided significance level of 0.05
Table 9. Efficacy comparison of sex assessed by the complete response in the delayed phase using the Modifed Intent To Treat population—MEC Trial
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Control p-value | |
|---|---|---|---|---|
| Women | ||||
| Men | ||||
| Control | 536 | 363 (59.3) | 9.1 | 0.002* |
| Rolapitant | 531 | 363 (68.4) | ||
| Control | 130 | 92 (70.8) | 12.2 | 0.018* |
| Rolapitant | 135 | 112 (83) | ||
FDA Statistical Review
*: Significant at two-sided significance level of 0.05
Table 10. Efficacy comparison of Race groups assessed by the complete response in the delayed phase using the Modifed Intent To Treat population—MEC Trial
| n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Control p-value | |
|---|---|---|---|---|
| Whites | ||||
| Non-whites | ||||
| Control | 512 | 327 (63.9) | 10.6 | 0.0003* |
| Rolapitant | 508 | 378 (74.4) | ||
| Control | 154 | 83 (53.9) | 7.5 | 0.18 |
| Rolapitant | 158 | 97 (61.4) | ||
FDA Statistical Review
*: Significant at two-sided significance level of 0.05
Table 11. Efficacy comparison of Age groups assessed by the complete response in the delayed phase using the Modifed Intent To Treat population—MEC Trial
| Age Group | n | Number (%) of Patients Responding | Rolapitant vs. Percent Difference | Control p-value |
|---|---|---|---|---|
| Age <> | ||||
| Age ≥65 | ||||
| Control | 470 | 286 (61) | 9 | 0.002* |
| Rolapitant | 495 | 348 (70) | ||
| Control | 196 | 124 (63) | 11 | 0.024* |
| Rolapitant | 171 | 127 (74) | ||
FDA Statistical Review
*: Significant at two-sided significance level of 0.05
What are the possible side effects?
The most common side effects are low white cell blood count, poor appetite, hiccups, and dizziness.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions seen in any patient who received at least one dose of VARUBI. Table 3 summarizes adverse reactions seen in the two HEC trials, and Table 4 summarizes adverse reactions seen in the MEC trial.
Table 3. Adverse Reactions Occurring at ≥ 3% in the Group and at Higher Rate than Control (HEC trials)
| Varubi * N = 624 | Control ** N = 627 | |
|---|---|---|
| Neutropenia | 9% | 8% |
| Hiccups | 5% | 4% |
| Abdominal Pain | 3% | 2% |
*Varubi, Dexamethasone, and 5HT3 Receptor Antagonist
**Placebo, Dexamethasone, and 5HT3 Receptor Antagonist
Source: Prescribing Information, Section 6, Table 2
Table 4. Adverse Reactions Occurring at ≥ 3% in the Group and at Higher Rate than Control (MEC trial)
| Varubi * N = 670 | Control** N = 674 | |
|---|---|---|
| Decreased appetite | 9% | 7% |
| Neutropenia | 7% | 6% |
| Dizziness | 6% | 4% |
| Dyspepsia | 4% | 2% |
| Urinary tract infection | 4% | 3% |
| Stomatitis | 4% | 2% |
| Anemia | 3% | 2% |
*Varubi, Dexamethasone, and 5HT3 Receptor Antagonist
**Placebo, Dexamethasone, and 5HT3 Receptor Antagonist
Source: Prescribing Information, Section 6, Table 3
Were there any differences in side effects among sex, race and age?
- Sex: VARUBI had a similar side effect profile in men and women.
- Race: The number of patients in the non-white subgroups was limited. Therefore, differences in side effects among races could not be concluded.
- Age: VARUBI had a similar side effect profile in patients below 65 years of age and those 65 years and above.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
According to Prescribing Information (Section 8.8), no overall differences in safety or efficacy were reported between the elderly subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
According to the Division Director Summary Review and an Addendum to the Clinical Review, there were no clinically meaningful differences in side effects between men and women. In addition, the size of non-white subgroups limited conclusions regarding side effects among subgroups.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved VARUBI based on evidence from 3 clinical trials of 2595 cancer patients who were receiving chemotherapies known to cause nausea and vomiting. The trials were conducted in Europe, North, Central and South America, Asia and South Africa.
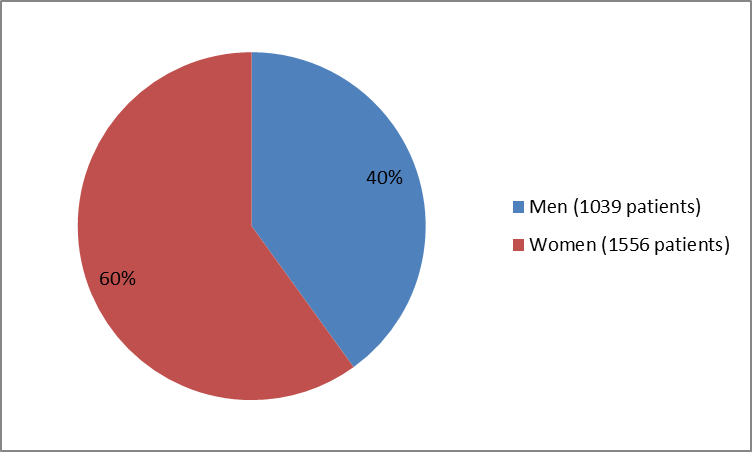
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Source: Company Trial Data
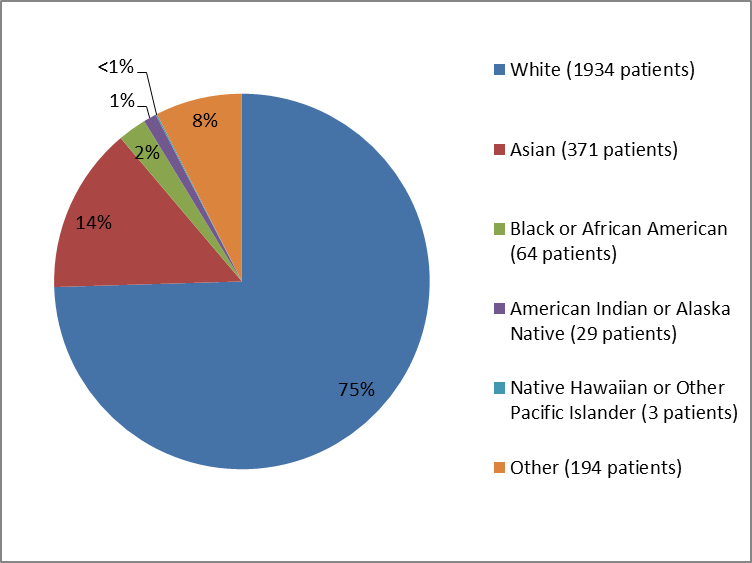
The figure and table below summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
<1%=less than="">
Source: Company Trial Data
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 1934 | 75% |
| Asian | 371 | 14% |
| Black or African American | 64 | 2% |
| American Indian or Alaska Native | 29 | 1% |
| Native Hawaiian or Other Pacific Islander | 3 | less than 1% |
| Other | 194 | 8% |
Source: Company Trial Data
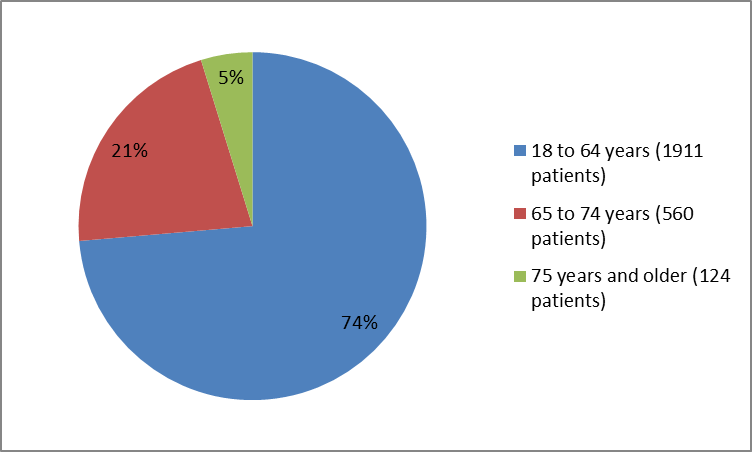
The figure below sumarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Source: Company Trial Data
Who participated in the trials?
The table below summarizes baseline characteristics (demographics) for patients participating in the trials, who were included in the safety assessment of VARUBI.
Table 5. Baseline Demographics
| Demographic Parameters | HEC Trials (N=1251) | MEC trial (N=1344) | Total (N=2595) n (%) | |||
|---|---|---|---|---|---|---|
| Varubi (N=624) n (%) | Control (N=627) n (%) | Varubi (N=670) n (%) | Control (N=674) n (%) | |||
| Sex | ||||||
| Age | ||||||
| Age Group | ||||||
| Race | ||||||
| Ethnicity | ||||||
| Region | ||||||
| Male | 385 (62) | 387 (62) | 135 (20) | 132 (20) | 1039 (40) | |
| Female | 239 (38) | 240 (38) | 535 (80) | 542 (80) | 1556 (60) | |
| Mean years (SD) | 57.0 (10.67) | 57.3 (10.86) | 56.8 (11.66) | 56.7 (11.99) | 56.9 (11.32) | |
| Median (years) | 58 | 58 | 58 | 56 | 58 | |
| Min, Max (years) | 20, 86 | 18, 90 | 22, 86 | 22, 88 | 18, 90 | |
| =18 - 64 years | 473 (76) | 464 (74) | 498 (74) | 476 (71) | 1911 (74) | |
| 65-74 years | 134 (21) | 142 (23) | 131 (20) | 153 (22) | 560 (21) | |
| =75 years | 17 (3) | 21( 3) | 41 (6) | 45 (7) | 124 (5) | |
| White | 456 (73) | 446 (71) | 512 (76) | 520 (77) | 1934 (75) | |
| Black or African American | 5 (1) | 6 (1) | 24 (4) | 29 (4) | 64 (3) | |
| Asian | 96 (15) | 99( 16) | 92 (14) | 84 (13) | 371 (14) | |
| American Indian or Alaska Native | 7 (1) | 9 (1) | 7 (1) | 6 (1) | 29 (1) | |
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 1 (<> | 2 (<> | 3 (<> | |
| Other | 60 (10) | 67 (11) | 34 (5) | 33 (5) | 194 (8) | |
| Hispanic or Latino | 119 (19) | 122 (20) | 77 (12) | 70 (10) | 388 (15) | |
| Not Hispanic or Latino | 505 (81) | 505 (80) | 588 (88) | 601 (90) | 2199 (85) | |
| North America | 63 (10) | 69 (11) | 216 (32) | 229 (34) | 577 (22) | |
| Central/South America | 114 (18) | 121 (19) | 31 (5) | 32 (5) | 298 (12) | |
| Europe | 339 (54) | 333 (53) | 316 (47) | 307 (46) | 1295 (50) | |
| Asia/South Africa | 108 (17) | 104 (17) | 107 (16) | 106 (16) | 425 (16) | |
Source: Company Trial Data
How were the trials designed?
There were three trials that evaluated the benefits and side effects of. In two trials patients were receiving chemotherapy which is known to cause nausea and vomiting often, and in one trial they were receiving chemotherapy which causes nausea and vomiting less often. Patients in all three trials were randomly assigned to receive either or placebo drug in addition to their regular medications for nausea and vomiting. Neither the patients nor the health care providers knew which treatment was being given until after the trial was complete.
The trials counted the number of patients who had no vomiting and used no medications for nausea or vomiting from 25 to 120 hours after the first day of the first cycle of chemotherapy, and compared VARUBI and placebo group.
How were the trials designed?
VARUBI for Highly Emetogenic Chemotherapy (HEC) was studied in two multicenter, randomized, double-blind, controlled clinical trials. regimen (, intravenous granisetron and dexamethasone) was compared with control therapy (placebo, intravenous granisetron and dexamethasone) in patients receiving a chemotherapy regimen that included cisplatin >60 mg/m2.
VARUBI for Moderately Emetogenic Chemotherapy (MEC) was studied in one multicenter, randomized, double-blind, controlled clinical trial. While this was considered a MEC trial, approximately half of the trial patients were receiving a combination of anthracycline and cyclophosphamide, which is considered HEC. regimen (, oral granisetron, and dexamethasone) was compared with control therapy (placebo, oral granisetron, and dexamethasone).
Trial drug was given 1-2 hours prior to chemotherapy Day 1.
The primary endpoint in all three trials was complete response defined as no vomiting and no use of additional medication for symptoms of nausea or vomiting, during the delayed phase (25 to 120 hours after the start of the first cycle of chemotherapy).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.