Drug Trials Snapshots: VEOPOZ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the VEOPOZ Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
VEOPOZ (pozelimab-bbfg)
Regeneron Pharmaceuticals, Inc.
Approval date: August 18, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VEOPOZ is used to treat adults and pediatric patients 1 year of age and older with CD55 deficient protein-losing enteropathy (PLE), also known as CHAPLE disease.
CHAPLE—which stands for complement hyperactivation, angiopathic thrombosis, and protein-losing enteropathy—is an inherited immune disease that causes the complement system (the part of your immune system that defends the body against injury and foreign invaders like bacteria and viruses) to become overactive. It is caused by mutations of the complement regulator CD55 gene, which can lead to the complement system attacking the body’s own cells.
Symptoms can include abdominal pain, nausea, vomiting, diarrhea, loss of appetite, weight loss, impaired growth, and edema (swelling). Severe thrombotic vascular occlusions (blockage of blood vessels) can also occur among patients with CHAPLE disease, which can be life-threating.
How is this drug used?
VEOPOZ is given by a health care professional as a single 30 mg/kg loading dose by intravenous infusion. Starting one week after the loading dose, patients will receive a dose (based upon body weight) once a week as a subcutaneous (under the skin) injection.
Who participated in the clinical trials?
The FDA approved VEOPOZ based on evidence from a single clinical trial of 10 patients with active CHAPLE disease who had hypoalbuminemia (persistent low albumin levels in the blood). Of the 10 patients, 7 enrolled at a site in Turkey, 2 in Thailand, and 1 in the United States.
How were the trials designed?
The efficacy and safety of VEOPOZ were assessed in a single-arm clinical trial that enrolled 10 patients with active CHAPLE disease. The primary outcomes were evaluated by assessing whether subjects achieved normal serum albumin concentrations levels by Week 12, and by assessing the number of albumin transfusions and total days of hospitalization over a period of 48 weeks. Patient outcomes were evaluated by comparing their condition after being treated with VEOPOZ to their state before starting VEOPOZ.
DEMOGRAPHICS SNAPSHOT
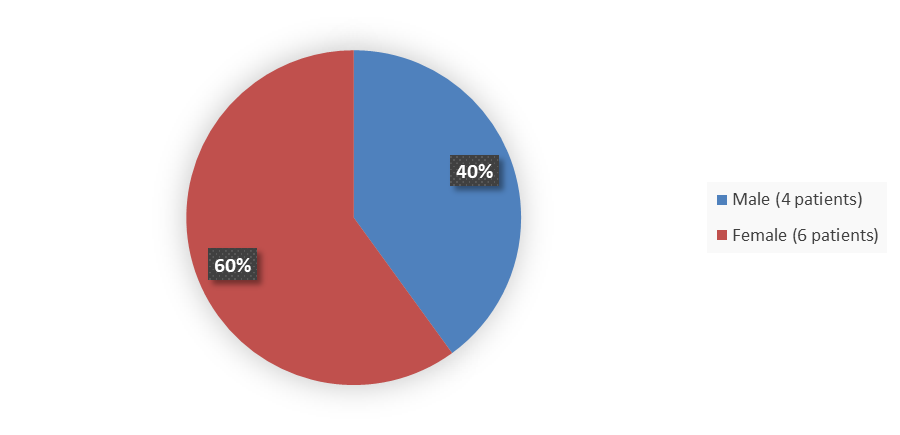
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of VEOPOZ.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
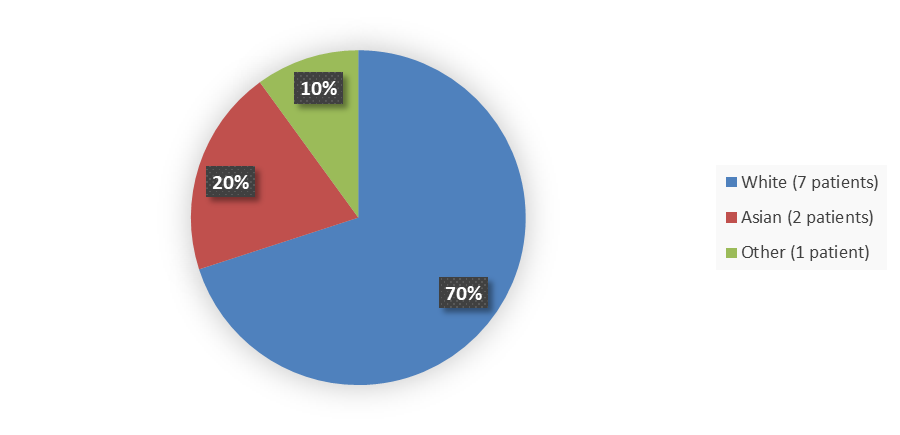
Figure 2 summarizes how many patients by race enrolled in the clinical trial used to evaluate the efficacy of VEOPOZ.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
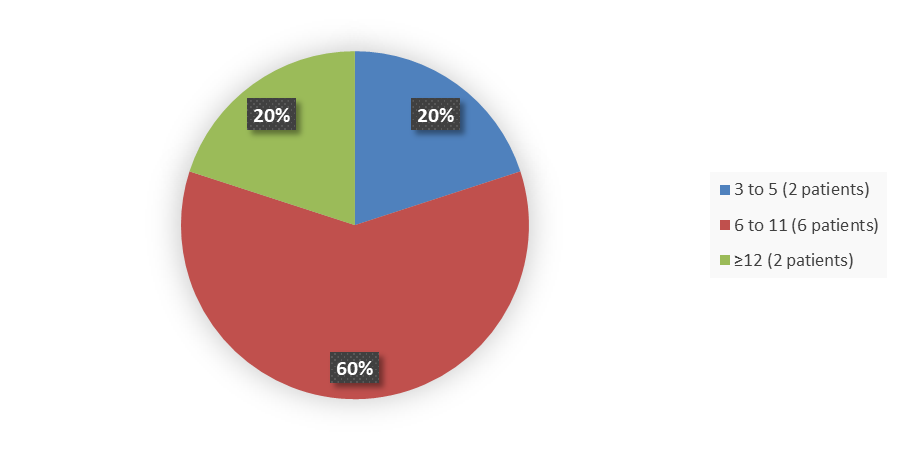
Figure 3 summarizes how many patients by age enrolled in the clinical trial used to evaluate the efficacy of VEOPOZ.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
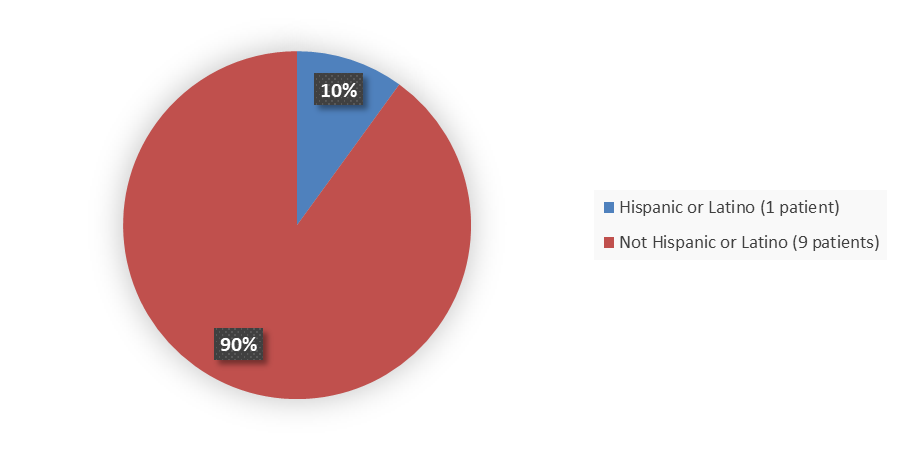
Figure 4 summarizes how many patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy of VEOPOZ.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Demographics Table
|
Demographics |
VEOPOZ N=10 |
|
Age group at screening, years, n (%) |
|
|
3 to 5 |
2 (20.0) |
|
6 to 11 |
6 (60.0) |
|
≥12 |
2 (20.0) |
|
Years of PLE from diagnosis to enrollment |
|
|
Mean (SD) |
7.3 (4.7) |
|
Median (range) |
6.0 (2.5, 15.9) |
|
Q1, Q3 |
3.8, 8.3 |
|
Sex, n (%) |
|
|
Male |
4 (40.0) |
|
Female |
6 (60.0) |
|
Race, n (%) |
|
|
White |
7 (70.0) |
|
Black or African American |
0 (0.0) |
|
Asian |
2 (20.0) |
|
American Indian or Alaska Native |
0 (0.0) |
|
Native Hawaiian or other Pacific Islander |
0 (0.0) |
|
Other |
1 (10.0) |
Source: Adapted from FDA Review
Abbreviations: PLE, protein-losing enteropathy; Q1, first quartile; Q3, third quartile; SD, standard deviation
What are the benefits of this drug?
The efficacy of VEOPOZ was evaluated in 10 patients by comparing serum albumin levels, the number of albumin transfusions, and the total days of hospitalization before and after starting VEOPOZ treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
After starting VEOPOZ treatment, all 10 patients achieved normalized serum albumin levels, decreased need for albumin transfusions, and fewer days of hospitalization. Additionally, all patients’ serum immunoglobulin G levels reached the normal range for age within the first 12 weeks of treatment.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age ?
The trial was too small to determine if there were any differences in how well the drug worked based on sex, race, or age.
What are the possible side effects?
VEOPOZ increases the risk of serious infections. Life-threatening and fatal meningococcal infections can occur, as well as other bacterial infections, and allergic reactions.
The most commonly reported side effects that occurred in two or more (20%) patients included upper respiratory tract infection; fracture; raised, red patches of skin that are often itchy (hives); and hair loss (alopecia). Additional side effects reported in one patient each (10%) included injection site reactions (including redness and irritation of the skin), bleeding of gums, abnormal blood tests (including increased uric acid, increased liver enzymes, metabolic acidosis), and blood and protein in the urine. Additionally, four patients reported high blood pressure readings above the normal range for age at multiple study visits.
Were there any differences in how side effects among sex, race, and age ?
The trial was too small to determine if there were any differences in side effects based on sex, race, or age.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION