Drug Trials Snapshots: VORANIGO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the VORANGIO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
VORANIGO (vorasidenib)
vo-rah-NEE-goh
Servier Pharmaceuticals, Inc
Original Approval date: August 6, 2024
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VORANIGO is an isocitrate dehydrogenase-1 (IDH1) and isocitrate dehydrogenase-2 (IDH2) inhibitor indicated for the treatment of adult and pediatric patients 12 years and older with grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation following surgery including biopsy, sub-total resection, or gross total resection.
How is this drug used?
VORANIGO a drug prescribed by health care professionals and taken each day by mouth.
Who participated in the clinical trials?
The FDA approved VORANIGO based on evidence from one clinical trial (INDIGO trial, NCT04164901) of 331 patients with IDH1-or IDH2-mutant grade 2 astrocytoma or oligodendroglioma with prior surgery including biopsy, sub-total resection, or gross total resection. The trial was conducted at 80 centers in the United States, Canada, France, Germany, Israel, Italy, Japan, Netherlands, Spain, Switzerland, and the United Kingdom. Of the 331 patients, 177 (53%) were from the United States.
The safety of VORANIGO was evaluated in 244 patients with astrocytoma or oligodendroglioma with susceptible IDH1 or IDH2 mutation from trials AG881-C-002 (NCT02481154, N=11), AG120-881-001 (NCT03343197, N=14), and INDIGO (NCT04164901, N=167 randomized patients and N=52 crossover patients).
How were the trials designed?
VORANIGO was evaluated in one randomized, multicenter, double-blind, placebo-controlled study of 331 patients (INDIGO). Eligible patients were required to have IDH1- or IDH2-mutant grade 2 astrocytoma or oligodendroglioma with prior surgery including biopsy, sub-total resection, or gross total resection. Patients were required to have measurable, non-enhancing disease; patients with centrally confirmed minimal, non-nodular, non-measurable enhancement were eligible. Patients who received prior anti-cancer treatment, including chemotherapy or radiation therapy were excluded. Patients were randomized to receive either VORANIGO 40 mg orally once daily or placebo orally once daily until disease progression or unacceptable toxicity. The trial measured the portion of time that patients did not have disease progression (progression free survival, PFS).
DEMOGRAPHICS SNAPSHOT
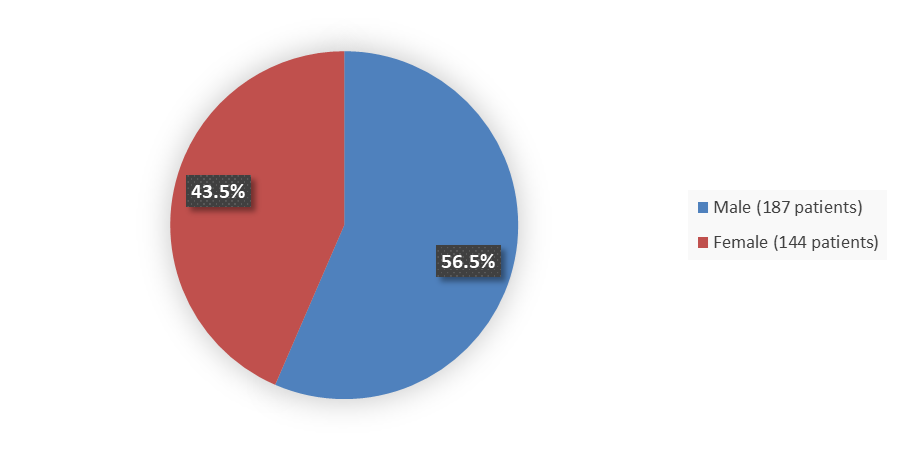
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of VORANIGO.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
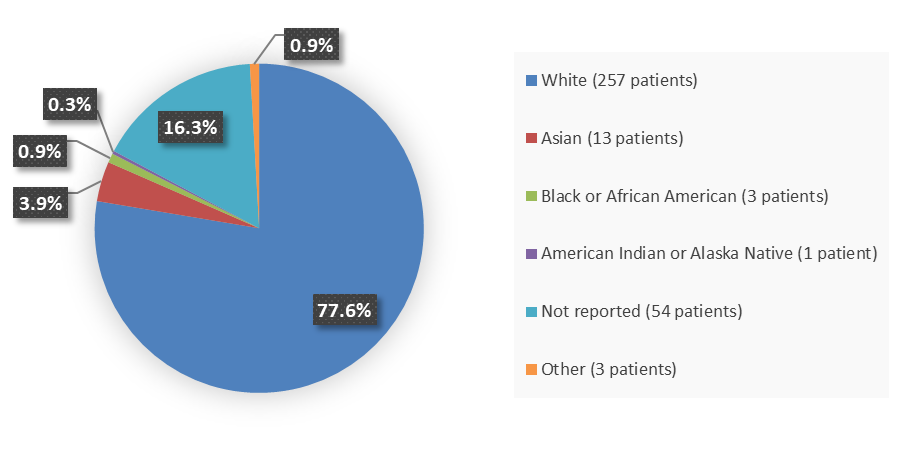
Figure 2 summarizes how many patients by race were enrolled in the clinical trial used to evaluate the efficacy of VORANIGO.
Figure 2. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
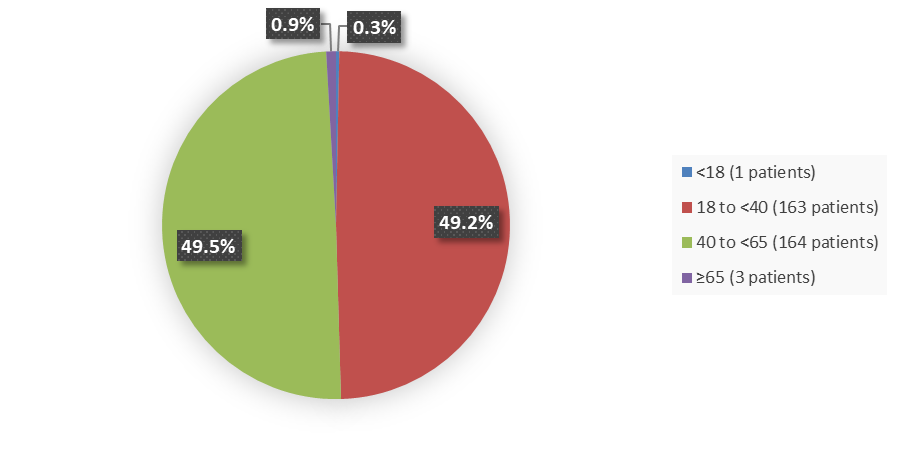
Figure 3 summarizes how many patients by age were enrolled in the clinical trial used to evaluate the efficacy of VORANIGO.
Figure 3. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
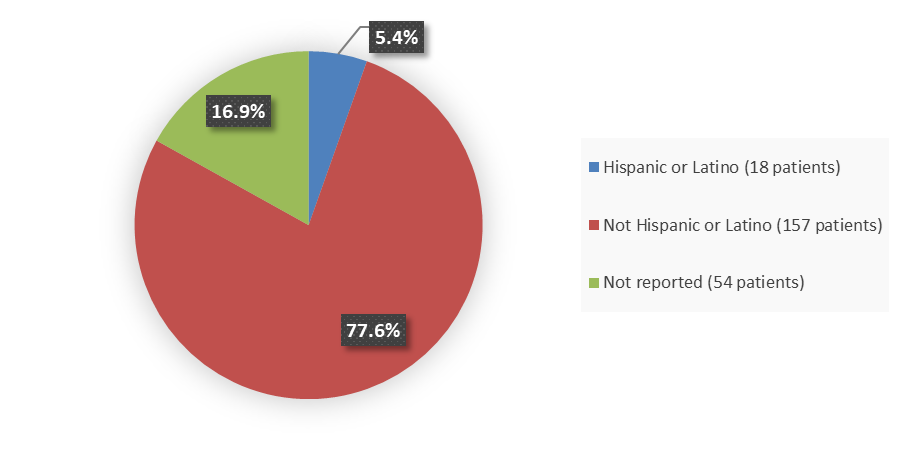
Figure 4 summarizes how many patients by ethnicity were enrolled in the clinical trial used to evaluate the efficacy of VORANIGO.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Demographics by Age, Race, Sex and Ethnicity
| Characteristics | VORANIGO | Placebo |
|---|---|---|
| Sex | ||
| Female | 67 (39.9) | 77 (47.2) |
| Male | 101 (60.1) | 86 (52.8) |
| Age, years | ||
| <18 | 0 | 1 (0.6) |
| 18 to <40 | 76 (45.2) | 87 (53.4) |
| 40 to <65 | 90 (53.6) | 74 (45.4) |
| >65 | 2 (1.2) | 1 (0.6) |
| Race | ||
| White | 125 (74.4) | 132 (81.0) |
| Asian | 5 (3.0) | 8 (4.9) |
| Black or African American | 2 (1.2) | 1 (0.6) |
| American Indian or Alaska Native | 1 (0.6) | 0 |
| Not reported | 33 (19.6) | 21 (12.9) |
| Other | 2 (1.2) | 1 (0.6) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 9 (5.4) | 9 (5.5) |
| Not Hispanic or Latino | 122 (72.6) | 135 (82.8) |
| Not reported | 37 (22.0) | 19 (11.7) |
Source: Adapted from FDA Review
What are the benefits of this drug?
VORANIGO was approved under FDA’s traditional approval program. In INDIGO, patients with IDH1- or IDH2-mutant grade 2 astrocytoma or oligodendroglioma with prior surgery including biopsy, sub-total resection, or gross total resection who received had VORANIGO had an improved overall PFS compared to placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2. Efficacy Results, Efficacy Population
| Progression-Free Survival | VORANIGO | Placebo |
|---|---|---|
| Disease progression, n (%) | 47 (28) | 88 (54) |
| Hazard ratio (95% CI)a | 0.39 (0.27, 0.56) | |
| p-valueb | 7lt; 0.0001 | |
Source: Adapted from VORANIGO Prescribing Information
a Stratified Cox proportional hazard model, stratified by 1p19q status and baseline tumor size
b Based on one-sided stratified log-rank test compared to the pre-specified α of 0.000359 (one-sided)
Abbreviations: CI, confidence interval
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: VORANIGO worked similarly in males and females.
- Race: VORANIGO worked similarly in White and Asian patients; differences among other races could not be determined because of the small number of patients of other races.
- Age: There was insufficient number of patients to determine whether VORANIGO worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3a. Efficacy Results by Race
Asian | Black or African American | White | Other | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Vorasidenib | Placebo | Vorasidenib | Placebo | Vorasidenib | Placebo | Vorasidenib | |
| Progression-free survival (months) | N=8 | N=5 | N=1 | N=2 | N=132 | N=132 | N=22 | N=36 |
| Median (95% CI) | 11.4 (2.9, NE) | NE (NE, NE | NE (NE, NE) | NE (NE, NE) | 11.1 (9.2, 13.8) | 19.4 (17.0, NE) | 11.2 (10.7, 13.9) | NE (10.6, NE) |
| Hazard ratio (95% CI) | 0.39 (0.26, 0.59) | 0.52 (0.23, 1.16) | ||||||
| P-value | 0.000001607 | 0.05261608 | ||||||
Source Primary NDA218784 Application submission, file 04-14-tables-figures: Table 14.2.1.8, data cut-off September 6, 2022.
Note: Progression-free survival (PFS) per BIRC refers to death or documented radiographic progressive disease (PD) as assessed by the BIRC per modified RANO-LGG.
Table 3b. Efficacy by Ethnicity
| Hispanic or Latino | Not Hispanic or Latino | Not reported | ||||
|---|---|---|---|---|---|---|
| Placebo | Vorasidenib | Placebo | Vorasidenib | Placebo | Vorasidenib | |
| Progression-free survival (months) | N=9 | N=9 | N=135 | N=122 | N=19 | N=37 |
| Median (95% CI) | 11.1 (5.5, 16.6) | 16.4 (10.2, NE) | 11.1 (9.2, 11.8) | 27.7 (17.0, NE) | 11.2 (10.7, 13.9) | NE (11.0, NE) |
| Hazard ratio (95% CI) | 0.64 (0.15, 2.70) | 0.38 (0.25, 0.57) | 0.47 (0.19, 1.17) | |||
| P-value | 0.26963657 | 8.74E-07 | 0.049254357 | |||
Source Primary NDA218784 Application submission, file 04-14-tables-figures: Table 14.2.1.9, data cut-off September 6, 2022.
Note: Progression-free survival (PFS) per BIRC refers to death or documented radiographic progressive disease (PD) as assessed by the BIRC per modified RANO-LGG.
Table 3c. Efficacy by Age
| Progression-free survival (months) | < 18 years | 18 to < 40 years | 40 to < 65 years | ≥ 65 years | ||||
|---|---|---|---|---|---|---|---|---|
| Placebo N=1 | Vorasidenib N=0 | Placebo N=87 | Vorasidenib N=76 | Placebo N=74 | Vorasidenib N=90 | Placebo N=1 | Placebo N=1 | |
| Median (95% CI) | NE (NE, NE) | NE (NE, NE) | 11.0 (8.6, 11.2) | 16.6 (14.3, NE) | 11.8 (11.1, 21.3) | 27.7 (18.5, NE) | NE (NE, NE) | 15.1 (11.0, 19.3) |
| Hazard ratio (95% CI) | 0.47 (0.29, 0.75) | 0.32 (0.18, 0.58) | ||||||
| P-value | 0.000632187 | 0.000028205 | ||||||
Source Primary NDA218784 Application submission, file 04-14-tables-figures: Table 14.2.1.11, data cut-off September 6, 2022.
Note: Progression-free survival (PFS) per BIRC refers to death or documented radiographic progressive disease (PD) as assessed by the BIRC per modified RANO-LGG.
Table 3d. Efficacy by Sex
| Progression-free survival (months) | Male | Female | ||
|---|---|---|---|---|
| Placebo N=86 | Vorasidenib N=101 | Placebo N=77 | Vorasidenib N=67 | |
| Median (95% CI) | 11.1 (8.6, 13.8) | 19.3 (16.5, NE) | 11.1 (11.0, 13.9) | NE (16.9, NE) |
| Hazard ratio (95% CI) | 0.39 (0.24, 0.64) | 0.41 (0.24, 0.70) | ||
| P-value | 0.000050338 | 0.000448507 | ||
Source Primary NDA218784 Application submission, file 04-14-tables-figures: Table 14.2.1.7, data cut-off September 6, 2022.
Note: Progression-free survival (PFS) per BIRC refers to death or documented radiographic progressive disease (PD) as assessed by the BIRC per modified RANO-LGG.
What are the possible side effects?
VORANIGO can cause serious side effects including hepatoxicity and embryo-fetal toxicity. The most common adverse reactions were fatigue, COVID-19, musculoskeletal pain, diarrhea, and seizure. The most common laboratory abnormalities were ALT increased, AST increased, GGT increased, and neutrophils decreased.
What are the possible side effects (results of trials used to assess safety)?
Table 3. Adverse Reactions (≥5%) in Patients With Grade 2 IDH1/2 Mutant Glioma Who Received VORANIGO Compared With Placebo in the INDIGO Trial
| Adverse Reactiona | VORANIGO,N=167 | Placebo, N=163 | ||
|---|---|---|---|---|
All Grades | Grades 3 or 4 | All Grades | Grades 3 or 4 | |
| Nervous system disorders | ||||
| Headacheb | 28 | 0 | 29 | 0.6 |
| Dizzinessc | 16 | 0 | 18 | 0 |
| Seizured | 16 | 4.2 | 15 | 3.7 |
| Musculoskeletal and connective tissue disorders | ||||
| Musculoskeletal paine | 26 | 0 | 25 | 1.8 |
| Gastrointestinal disorders | ||||
| Nausea | 22 | 0 | 23 | 0 |
| Diarrheaf | 25 | 0.6 | 17 | 0.6 |
| Vomiting | 7 | 0 | 10 | 0 |
| Decreased appetite | 9 | 0 | 3.7 | 0 |
| Constipation | 13 | 0 | 12 | 0 |
| Abdominal paing | 13 | 0 | 12 | 0 |
| Infections and infestations | ||||
| COVID-19 | 33 | 0 | 29 | 0 |
| General disorders | ||||
| Fatigueh | 37 | 0.6 | 36 | 1.2 |
Source: Adapted from FDA Review
a Adverse reactions are based on NCI CTCAE v5.0.
b Grouped term includes sinus headache, migraine, migraine with aura, postictal headache, ophthalmic migraine, and tension headache.
c Grouped term includes vertigo.
d Grouped term includes partial seizures, generalised tonic-clonic seizure, epilepsy, clonic convulsion, and simple partial seizures.
e Grouped term includes arthralgia, back pain, non-cardiac chest pain, pain in extremity, myalgia, neck pain, musculoskeletal chest pain, arthritis, and musculoskeletal stiffness.
f Grouped term includes feces soft and frequent bowel movements.
g Grouped term includes abdominal pain upper, abdominal discomfort, abdominal pain lower, abdominal tenderness, and epigastric discomfort.
h Grouped term includes asthenia.
Note: Some events (headache, dizziness, nausea, and vomiting) were excluded from the product labeling per FDA labeling guidelines, because they occurred more commonly in the placebo arm.
Abbreviations: IDH1/2, isocitrate dehydrogenase-1/2; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events
Table 4. Select Laboratory Abnormalities (≥5%) That Worsened From Baseline in Patients with Grade 2 IDH1/2 Mutant Glioma Who Received VORANIGO in the INDIGO Trial
Parameter | VORANIGO, N=167 | Placebo, N=163 | ||
|---|---|---|---|---|
All Gradesa %b | Gradesa 3 or 4 %b | All Gradesa %b | Gradesa 3 or 4 %b | |
| Chemistry | ||||
| Increased ALT | 59 | 10 | 25 | 0 |
| Increased AST | 46 | 4.8 | 20 | 0 |
| Increased creatinine | 11 | 0.6 | 7 | 0 |
| Decreased calcium | 10 | 0 | 7 | 0 |
| Increased glucosec | 10 | 0 | 4.3 | 0 |
| Increased GGT | 38 | 3 | 10 | 1.8 |
| Decreased phosphated | 8 | 0.6 | 4.9 | 0 |
| Increased potassium | 23 | 0.6 | 20 | 0 |
| Increased ALP | 10 | 1.2 | 7 | 0.6 |
| Hematology | ||||
| Increased hemoglobin | 13 | 0 | 3.1 | 0 |
| Decreased lymphocytes | 11 | 1.8 | 8 | 0.6 |
| Decreased leukocytes | 13 | 0.6 | 12 | 0.6 |
| Decreased neutrophils | 14 | 2.4 | 12 | 1.8 |
| Decreased platelets | 12 | 0 | 4.3 | 0 |
Source: VORANIGO Prescribing Information
a Based on NCI CTCAE v5.0.
b The denominator used to calculate percentages is N, the number of subjects in the Safety Analysis Set within each treatment group.
c Includes adverse reaction term hyperglycemia.
d Includes adverse reaction terms hypophosphatemia and blood phosphorus decreased.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similarly in White, Black or African American, and Asian patients; differences among other races could not be determined because of the small number of patients of other races.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
There were no significant differences in the clinical side effects observed in the INDIGO trial based on sex, race, and age groups.
Table 5. Adverse Events by Sex, Safety Population
Study INDIGO | ||||
|---|---|---|---|---|
Vorasidenib | Placebo | |||
Male | Female | Male | Female | |
| All treatment-emergent adverse events | 96 (95.0) | 62 (93.9) | 80 (93.0) | 72 (93.5) |
| Serious adverse events | 4 (4.0) | 7 (10.6) | 4 (4.7) | 4 (5.2) |
| Serious adverse events | 29 (28.7) | 21 (31.8) | 22 (25.6) | 15 (19.5) |
| Leading to discontinuation of VORANIGO | 2 (2.0) | 4 (6.1) | 1 (1.2) | 1 (1.3) |
Source: Adapted from FDA Review
The safety population includes all subjects from INDIGO who have received at least one dose of VORANIGO (without crossover). One participant in INDIGO was randomized to VORANIGO but did not receive any dose of VORANIGO and was not included.
A subject with multiple occurrences of an AE is counted only once in the AE category.
Abbreviations: N, number of patients in treatment arm; n, number of patients with adverse event; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
Table 6. Adverse Events by Age Group, Safety Population
Study INDIGO | ||||
|---|---|---|---|---|
Vorasidenib | Placebo | |||
Age < 65 | Age ≥ 65 | Age < 65 | Age ≥ 65 | |
| All treatment-emergent adverse events | 156 (94.5) | 2 (100) | 151 (93.2) | 1 (100) |
| Serious adverse events | 11 (6.7) | 0 | 8 (4.9) | 0 |
| Leading to dose interruption and/or reduction of VORANIGO | 50 (30.3) | 0 | 37 (22.8) | 0 |
| Leading to discontinuation of VORANIGO | 6 (3.6) | 0 | 2 (1.2) | 0 |
Source: Adapted from FDA Review
The safety population includes all subjects from INDIGO who have received at least one dose of VORANIGO (without crossover). One participant in INDIGO was randomized to VORANIGO but did not receive any dose of VORANIGO and was not included.
A subject with multiple occurrences of an AE is counted only once in the AE category.
Abbreviations: N, number of patients in treatment arm; n, number of patients with adverse event; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
Table 7. Adverse Events by Race, Safety Population
Study INDIGO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
Vorasidenib | Placebo | Vorasidenib | Placebo | Vorasidenib | Placebo | Vorasidenib | Placebo | Vorasidenib | Placebo | |
Black or African American | Black or African American | Other | Other | Asian | Asian | White | White | Unknown | Unknown | |
| All treatment-emergent adverse events | 2 (100) | 1 (100) | 3 (100) | 1 (100) | 5 (100) | 8 (100) | 118 (95.2) | 123 (93.2) | 30 (90.9) | 19 (90.5) |
| Serious adverse events | 0 | 1 (100) | 0 | 0 | 0 | 0 | 8 (6.5) | 6 (4.5) | 3 (9.1) | 1 (4.8) |
| Leading to dose interruption and/or reduction of VORANIGO | 0 | 1 (100) | 0 | 1 (100) | 4 (80.0) | 2 (25.0) | 35 (28.2) | 29 (22.0) | 11 (33.3) | 4 (19.0) |
| Leading to discontinuation of VORANIGO | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 3 (2.4) | 2 (1.5) | 2 (6.1) | 0 |
Source: Adapted from FDA Review
The safety population includes all subjects from INDIGO who have received at least one dose of VORANIGO (without crossover). One participant in INDIGO was randomized to VORANIGO but did not receive any dose of VORANIGO and was not included.
A subject with multiple occurrences of an AE is counted only once in the AE category.
Abbreviations: N, number of patients in treatment arm; n, number of patients with adverse event; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.