Drug Trials Snapshots: VYLOY
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the VYLOY Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
VYLOY (zolbetuximab-clzb)
(vye-LOY)
Astellas Pharma US, Inc.
Approval date: October 18, 2024
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
VYLOY is a claudin (CLDN) 18.2-directed cytolytic antibody indicated in combination with fluoropyrimidine- and platinum-containing chemotherapy for the treatment of adults with gastric or gastroesophageal junction adenocarcinoma whose tumors are CLDN18.2-positive and human epidermal growth factor receptor 2 (HER2)-negative that cannot be removed by surgery or has spread to other parts of the body (metastatic).
It should be used in patients who have not been previously treated with chemotherapy and other anticancer agents.
How is this drug used?
VYLOY is given as an intravenous infusion in combination with chemotherapy; depending on the type of chemotherapy, VYLOY is administered every two to three weeks.
Who participated in the clinical trials?
The FDA approved VYLOY based on evidence from two clinical trials, SPOTLIGHT (NCT03504397) and GLOW (NCT03653507), that enrolled 1,072 patients with CLDN18.2-positive and HER2-negative gastric or gastroesophageal junction adenocarcinoma. The trials were conducted at 408 sites in 30 of countries in Asia, Europe, North America, South America, and Australia. Of the 1,072 patients, 74 were from the United States. Both clinical trials were used to assess the efficacy and safety of VYLOY.
How were the trials designed?
The benefit and side effects of VYLOY administered in combination with two types of chemotherapy combinations called mFOLFOX6 or CAPOX were evaluated in two clinical trials of patients with previously untreated HER2-negative gastric or gastroesophageal cancer whose tumors were CLDN18.2-positive that spread to other parts of the body or that could not be removed by surgery.
How were the trials designed?
In the SPOTLIGHT trial, 565 patients were randomized to receive VYLOY plus mFOLFOX6 or placebo plus mFOLFOX6. Patients received up to 12 treatments of VYLOY or placebo and mFOLFOX6 and after 12 treatments, patients were allowed to continue treatment with VYLOY, 5-fluorouracil, and folinic acid alone until the disease progressed or the side effects became too toxic.
In the GLOW trial, 507 patients received either VYLOY combined with CAPOX or placebo in combination with CAPOX. Patients received up to eight treatments of VYLOY or placebo and CAPOX, and after eight treatments, patients were allowed to continue treatment with VYLOY and capecitabine alone until the disease progressed or the side effects became too toxic.
The benefit of VYLOY plus mFOLFOX6 or CAPOX was evaluated by measuring the length of time tumors did not grow or spread after start of treatment (progression-free survival or PFS) and the length of time that patients survived after start of treatment (overall survival).
DEMOGRAPHICS SNAPSHOT
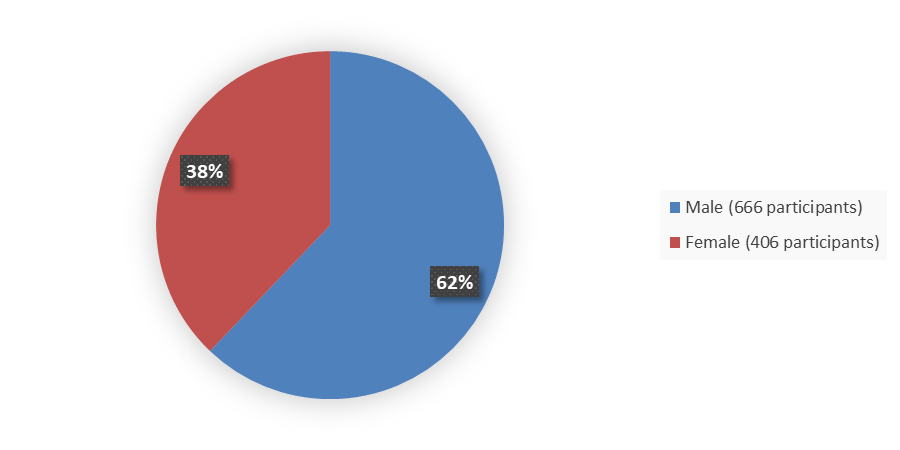
Figure 1 summarizes how many male and female patients were enrolled in the combined clinical trials used to evaluate the efficacy of VYLOY.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
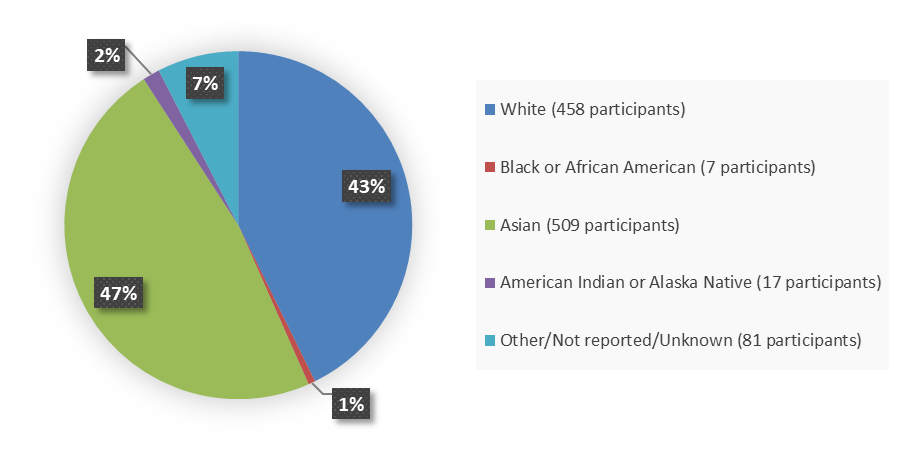
Figure 2 summarizes how many patients by race were enrolled in the combined clinical trials used to evaluate the efficacy of VYLOY.
Figure 2. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
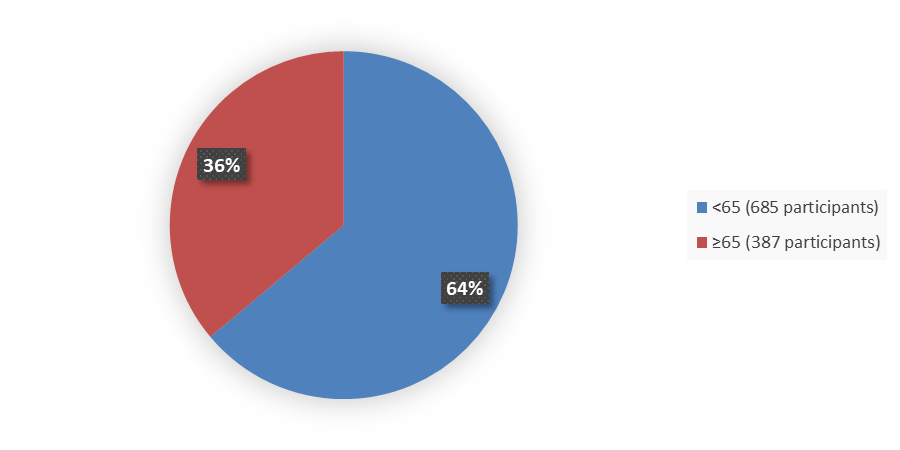
Figure 3 summarizes how many patients by age were enrolled in the combined clinical trials used to evaluate the efficacy of VYLOY.
Figure 3. Baseline Demographics by Age, Efficacy Population
Source: FDA Analysis
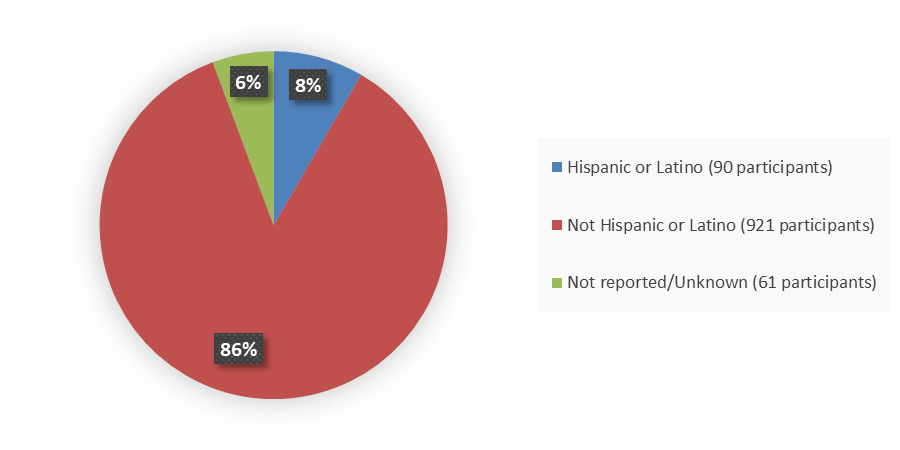
Figure 4 summarizes how many patients by ethnicity were enrolled in the combined clinical trials used to evaluate the efficacy of VYLOY.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics of Efficacy Trials, Efficacy Population
Demographic Subgroup |
SPOTLIGHT | GLOW | ||
|---|---|---|---|---|
| VYLOY + mFOLFOX6 N=283 |
Placebo + mFOLFOX6 N=282 |
VYLOY + CAPOX N=254 |
Placebo + CAPOX N=253 |
|
| Sex, n (%) | ||||
| Female | 107 (38) | 107 (38) | 95 (37) | 97 (38) |
| Male | 176 (62) | 175 (62) | 159 (63) | 156 (62) |
| Age, years | ||||
| Median (min, max) | 62.0 (27, 83) | 60.0 (20, 86) | 61.0 (22, 82) | 59.0 (21, 83) |
| Age group, n (%) | ||||
| <65 | 171 (60) | 174 (62) | 167 (66) | 173 (68) |
| ≥65 | 112 (40) | 108 (38) | 87 (34) | 80 (32) |
| Race, n (%) | ||||
| Asian | 96 (34) | 97 (34) | 158 (62) | 158 (62) |
| White | 140 (49) | 134 (48) | 94 (37) | 90 (36) |
| American Indian or Alaska Native | 9 (3.2) | 8 (2.8) | 0 | 0 |
| Black or African American | 5 (1.8) | 2 (0.7) | 0 | 0 |
| Other | 11 (3.9) | 12 (4.3) | 0 | 0 |
| Missing | 22 (8) | 29 (10) | 2 (0.8) | 5 (2) |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 36 (13) | 37 (13) | 10 (3.9) | 7 (2.8) |
| Not Hispanic or Latino | 225 (80) | 213 (76) | 242 (95) | 241 (95) |
| Missing | 22 (8) | 32 (11) | 2 (0.8) | 5 (2.8) |
Source: FDA Analysis
What are the benefits of this drug?
The benefit of VYLOY was evaluated by measuring the length of time tumors did not grow or spread after start of treatment (PFS) and the length of time that patients survived after start of treatment (overall survival). In the SPOTLIGHT study, VYLOY in combination with mFOLFOX6 lowered the risk of progression or death by 25%. In the GLOW study, VYLOY in combination with CAPOX lowered the risk of progression or death by 31%.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2. Efficacy Results, Efficacy Population
Efficacy Parameter |
SPOTLIGHT | GLOW | ||
|---|---|---|---|---|
| VYLOY + mFOLFOX6 N=283 |
Placebo + mFOLFOX6 N=282 |
VYLOY + CAPOX N=254 |
Placebo + CAPOX N=253 |
|
| Progression free survival | ||||
| Number of patients with events, n (%) | 146 (51.6) | 167 (59.2) | 137 (53.9) | 172 (68.0) |
| Median in months (95% CI)1 | 10.6 (8.9, 12.5) | 8.7 (8.2, 10.3) | 8.2 (7.5, 8.8) | 6.8 (6.1, 8.1) |
| Hazard ratio (95% CI)2,3 | 0.751 (0.598, 0.942) | 0.687 (0.544, 0.866) | ||
| 1-sided p-value2,4 | 0.0066 | 0.0007 | ||
| Overall survival | ||||
| Number of patient deaths, n (%) | 149 (52.7) | 177 (62.8) | 144 (56.7) | 174 (68.8) |
| Median in months (95% CI)1 | 18.2 (16.4, 22.9) | 15.5 (13.5, 16.5) | 14.4 (12.3, 16.5) | 12.2 (10.3, 13.7) |
| Hazard ratio (95% CI)2,3 | 0.750 (0.601, 0.936) | 0.771 (0.615, 0.965) | ||
| 1-sided p-value2,4 | 0.0053 | 0.0118 | ||
| Objective response rate (CR + PR)5 | ||||
| ORR, % (95% CI)6 | 40.3 (34.5, 46.3) | 39.7 (34.0, 45.7) | 32.3 (26.6, 38.4) | 31.2 (25.6, 37.3) |
| Complete response rate, n (%) | 14 (4.9) | 8 (2.8) | 6 (2.4) | 2 (0.8) |
| Partial response rate, n (%) | 100 (35.3) | 104 (36.9) | 76 (29.9) | 77 (30.4) |
| Duration of response | N=114 | N=112 | N=82 | N=79 |
| Median in months (95% CI) | 10.3 (8.3, 10.9) | 10.5 (7.7, 13.3) | 8.3 (6.3, 11.4) | 6.2 (6.0, 7.6) |
Source: VYLOY Prescribing Information
1 Based on Kaplan-Meier estimate.
2 Stratification factors were region, number of metastatic sites and prior gastrectomy from Interactive Response Technology.
3 Based on a stratified Cox proportional hazards model. 4 Based on a 1-sided stratified log-rank test. 5 Based on confirmed response. 6 Based on binomial distribution (Clopper-Pearson).
Abbreviations: CI, confidence interval; CR, complete response; ORR, overall response rate; PR, partial response
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: The effect of VYLOY was similar for females and males.
- Race: The observed effect of VYLOY was larger for Asian patients than for White patients. Because of limited data, this difference may be due to chance. The number of American Indian or Alaska Native and Black or African American patients was small; therefore, differences in how the drug worked in these races could not be determined.
- Age: The effect of VYLOY was similar in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3. Progression-Free Survival by Subgroup, Efficacy Population
Subgroup |
SPOTLIGHT | GLOW | ||||
|---|---|---|---|---|---|---|
| VYLOY + mFOLFOX6 N=283 n/Ns (%) |
Placebo + mFOLFOX6 N=282 n/Ns(%) |
Hazard Ratio (95% CI) | VYLOY + CAPOX N=254 n/Ns (%) |
Placebo + CAPOX N=253 n/Ns (%) |
Hazard Ratio (95% CI) | |
| Sex | ||||||
| Female | 51/107 (47.7) | 61/107 (57.0) | 0.711 (0.488, 1.034) | 45/95 (47.4) | 62/97 (63.9) | 0.700 (0.474, 1.035) |
| Male | 95/176 (54.0) | 106/175 (60.6) | 0.776 (0.587, 1.024) | 92/159 (57.9) | 110/156 (70.5) | 0.679 (0.513, 0.898) |
| Race | ||||||
| Asian | 47/96 (49.0) | 51/97 (52.6) | 0.527 (0.352, 0.788) | 83/158 (52.5) | 109/158 (69.0) | 0.587 (0.440, 0.785) |
| White | 77/140 (55.0) | 82/134 (61.2) | 0.930 (0.681, 1.271) | 52/94 (55.3) | 58/90 (64.4) | 0.918 (0.629, 1.339) |
| Age, years | ||||||
| <65 | 88/171 (51.5) | 101/174 (58.0) | 0.749 (0.562, 0.998) | 88/167 (52.7) | 124/173 (71.7) | 0.599 (0.454, 0.790) |
| ≥65 | 58/112 (51.8) | 66/108 (61.1) | 0.748 (0.524, 1.067) | 49/87 (56.3) | 48/80 (60.0) | 0.934 (0.624, 1.397) |
Source: FDA Analysis
Abbreviations: CI, confidence interval; N, number of patients in treatment arm; n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
Table 4. Overall Survival by Subgroup, Efficacy Population
Subgroup |
SPOTLIGHT | GLOW | ||||
|---|---|---|---|---|---|---|
| VYLOY + mFOLFOX6 N=283 n/Ns (%) |
Placebo + mFOLFOX6 N=282 n/Ns (%) |
Hazard Ratio (95% CI) | VYLOY + CAPOX N=254 n/Ns (%) |
Placebo + CAPOX N=253 n/Ns (%) |
Hazard Ratio (95% CI) | |
| Sex | ||||||
| Female | 51/107 (47.7) | 64/107 (59.8) | 0.726 (0.502, 1.049) | 50/95 (52.6) | 60/97 (61.9) | 0.722 (0.494, 1.054) |
| Male | 98/176 (55.7) | 113/175 (64.6) | 0.760 (0.579, 0.999) | 94/159 (59.1) | 114/156 (73.1) | 0.779 (0.593, 1.025) |
| Race | ||||||
| Asian | 49/96 (51.0) | 65/97 (67.0) | 0.572 (0.393, 0.832) | 89/158 (56.3) | 110/158 (69.6) | 0.678 (0.512, 0.898) |
| White | 81/140 (57.9) | 81/134 (60.4) | 0.948 (0.696, 1.291) | 53/94 (56.4) | 60/90 (66.7) | 0.891 (0.615, 1.291) |
| Age, years | ||||||
| <65 | 83/171 (48.5) | 106/174 (60.9) | 0.719 (0.539, 0.959) | 87/167 (52.1) | 120/173 (69.4) | 0.669 (0.507, 0.882) |
| ≥65 | 66/112 (58.9) | 71/108 (65.7) | 0.782 (0.557, 1.097) | 57/87 (65.5) | 54/80 (67.5) | 0.914 (0.628, 1.330) |
Source: FDA Analysis
Abbreviations: CI, confidence interval; N, number of patients in treatment arm; n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
What are the possible side effects?
VYLOY may cause serious side effects, including hypersensitivity reactions (anaphylaxis and infusion-related reactions) and severe nausea and vomiting.
The most common side effects for VYLOY in combination with mFOLFOX6 or CAPOX were nausea, vomiting, fatigue, decreased appetite, diarrhea, peripheral sensory neuropathy, abdominal pain, constipation, decreased weight, hypersensitivity reactions, and fever.
What are the possible side effects (results of trials used to assess safety)?
Table 5. Adverse Reactions (≥15%) in Patients Treated With VYLOY in SPOTLIGHT With a Difference Between Arms of ≥5% Compared to Placebo, Safety Population
| Adverse Reaction | VYLOY + mFOLFOX6 N=279 |
Placebo + mFOLFOX6 N=278 |
|||
|---|---|---|---|---|---|
| All Grades % |
Grade 3 or 4 % |
All Grades % |
Grade 3 or 4 % |
||
| Gastrointestinal disorders | |||||
| Nausea | 82 | 16 | 61 | 7 | |
| Vomiting | 67 | 16 | 36 | 6 | |
| Metabolism and nutrition disorders | |||||
| Decreased appetite | 47 | 6 | 34 | 3.2 | |
| General disorders and administration site conditions | |||||
| Peripheral edema | 18 | 0.7 | 9 | 0 | |
Source: VYLOY Prescribing Information
Table 6. Adverse Reactions (≥15%) in Patients Treated With VYLOY in GLOW With a Difference Between Arms of ≥5% Compared to Placebo, Safety Population
| Adverse Reaction | VYLOY + CAPOX N=254 |
Placebo + CAPOX N=249 |
||
|---|---|---|---|---|
| All Grades % |
Grade 3 or 4 % |
All Grades % |
Grade 3 or 4 % |
|
| Gastrointestinal disorders | ||||
| Nausea | 69 | 9 | 50 | 2.4 |
| Vomiting | 66 | 12 | 31 | 3.6 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 41 | 7 | 34 | 1.6 |
| Blood and lymphatic system disorders | ||||
| Neutropenia | 20 | 7 | 14 | 2.8 |
| Investigations | ||||
| Weight decreased | 20 | 0.4 | 10 | 0.4 |
Source: VYLOY Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar in White and Asian patients. The number of patients of other races was small; therefore, differences in the occurrence of side effects in other races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 7. Select Adverse Events by Sex in SPOTLIGHT and GLOW, Safety Population
Adverse Event |
VYLOY + mFOLFOX6 (SPOTLIGHT) |
Placebo + mFOLFOX6 (SPOTLIGHT) |
VYLOY + CAPOX (GLOW) |
Placebo + CAPOX (GLOW) |
||||
|---|---|---|---|---|---|---|---|---|
| Male N=173 n (%) |
Female N=106 n (%) |
Male N=173 n (%) |
Female N=105 n (%) |
Male N=160 n (%) |
Female N=94 n (%) |
Male N=152 n (%) | Female N=97 n (%) |
|
| All TEAEs | 173 (100) | 105 (99) | 172 (99) | 105 (100) | 158 (99) | 93 (99) | 149 (98) | 95 (98) |
| Nausea | 137 (79) | 93 (88) | 106 (61) | 63 (60) | 108 (68) | 66 (70) | 72 (47) | 53 (55) |
| Vomiting | 111 (64) | 77 (73) | 55 (32) | 44 (42) | 101 (63) | 67 (71) | 41 (27) | 36 (37) |
| Abdominal pain | 56 (32) | 39 (37) | 64 (37) | 40 (38) | 37 (23) | 22 (23) | 36 (24) | 27 (28) |
| Fatigue | 97 (56) | 61 (58) | 92 (53) | 55 (52) | 59 (37) | 36 (38) | 57 (38) | 36 (37) |
| Infusion-related reaction | 3 (1.7) | 3 (2.8) | 4 (2.3) | 1 (1) | 6 (3.8) | 8 (9) | 2 (1.3) | 0 |
| Anemia | 51 (29) | 49 (46) | 59 (34) | 55 (52) | 48 (30) | 42 (45) | 56 (37) | 35 (36) |
| Neutropenia | 56 (32) | 47 (44) | 53 (31) | 41 (39) | 25 (16) | 25 (27) | 19 (13) | 16 (16) |
| Hypokalemia | 27 (16) | 23 (22) | 21 (12) | 20 (19) | 18 (11) | 18 (19) | 19 (13) | 17 (18) |
| Hypoalbuminemia | 31 (18) | 12 (11) | 7 (4) | 10 (10) | 37 (23) | 20 (21) | 26 (17) | 9 (9) |
Source: FDA Analysis
Abbreviations: TEAE, treatment-emergent adverse event
Table 8. Select Adverse Events by Race in SPOTLIGHT and GLOW, Safety Population
Adverse Event |
VYLOY + mFOLFOX6 (SPOTLIGHT) |
Placebo + mFOLFOX6 (SPOTLIGHT) |
VYLOY + CAPOX (GLOW) |
Placebo+ CAPOX (GLOW) |
||||
|---|---|---|---|---|---|---|---|---|
| Asian N=95 n (%) |
Other1 N=184 n (%) |
Asian N=94 n (%) |
Other1 N=155 n (%) |
Asian N=158 n (%) |
Other1 N=96 n (%) |
Asian N=156 n (%) |
Other1 N=93 n (%) |
|
| All TEAEs | 95 (100) | 182 (99) | 93 (99) | 155 (100) | 158 (100) | 93 (97) | 151 (97) | 93 (100) |
| Nausea | 83 (87) | 147 (80) | 63 (67) | 88 (57) | 114 (72) | 60 (63) | 77 (49) | 48 (52) |
| Vomiting | 61 (64) | 127 (69) | 31 (33) | 56 (36) | 109 (69) | 59 (62) | 45 (29) | 32 (34) |
| Abdominal pain | 15 (16) | 62 (34) | 32 (34) | 59 (38) | 37 (23) | 22 (23) | 37 (24) | 26 (28) |
| Fatigue | 44 (46) | 114 (62) | 30 (32) | 98 (63) | 54 (34) | 41 (43) | 53 (34) | 40 (43) |
| Infusion-related reaction | 1 (1.1) | 5 (2.7) | 4 (4.3) | 1 (0.6 | 6 (3.8) | 8 (8) | 0 | 2 (2.2) |
| Anemia | 36 (38) | 64 (35) | 32 (34) | 58 (37) | 60 (38) | 30 (31) | 66 (42) | 25 (27) |
| Neutropenia | 22 (23) | 87 (47) | 17 (18) | 69 (45) | 21 (13) | 29 (30) | 10 (6) | 25 (27) |
| Hypokalemia | 17 (18) | 28 (15) | 9 (10) | 30 (19) | 29 (18) | 7 (7) | 26 (17) | 10 (11) |
| Hypoalbuminemia | 15 (16) | 33 (18) | 6 (6) | 9 (6) | 45 (29) | 12 (13) | 30 (19) | 5 (5) |
Source: FDA Analysis
1 Other includes: White, American Indian or Alaska Native, Black or African American, other, and missing
Abbreviations: TEAE, treatment-emergent adverse event
Table 9. Select Adverse Events by Age in SPOTLIGHT and GLOW, Safety Population
Adverse Event |
VYLOY + mFOLFOX6 (SPOTLIGHT) |
Placebo + mFOLFOX6 (SPOTLIGHT) | VYLOY + CAPOX (GLOW) |
Placebo + CAPOX (GLOW) | ||||
|---|---|---|---|---|---|---|---|---|
| <65 Years N=168 n (%) |
≥65 Years N=111 n (%) |
<65 Years N=170 n (%) |
≥65 Years N=108 n (%) |
<65 Years N=167 n (%) |
≥65 Years N=87 n (%) |
<65 Years N=170 n (%) |
≥65 Years N=79 n (%) |
|
| All TEAEs | 168 (100) | 110 (100) | 169 (99) | 108 (100) | 165 (99) | 86 (99) | 166 (98) | 70 (99) |
| Nausea | 141 (84) | 89 (81) | 114 (67) | 55 (51) | 118 (71) | 56 (64) | 92 (54) | 33 (42) |
| Vomiting | 123 (73) | 65 (59) | 71 (42) | 28 (26) | 117 (70) | 51 (59) | 57 (34) | 20 (25) |
| Abdominal pain | 62 (37) | 33 (30) | 67 (39) | 37 (34) | 39 (23) | 20 (23) | 48 (28) | 15 (19) |
| Fatigue | 93 (55) | 65 (59) | 93 (55) | 54 (50) | 60 (36) | 35 (40) | 67 (39) | 35 (44) |
| Infusion-related reaction | 2 (1.2) | 4 (3.6) | 4 (2.4) | 1 (1) | 8 (4.8) | 6 (7) | 2 (1.2) | 0 |
| Anemia | 59 (35) | 41 (37) | 65 (38) | 39 (36) | 63 (38) | 27 (31) | 62 (36) | 29 (37) |
| Neutropenia | 58 (35) | 44 (40) | 57 (34) | 37 (34) | 29 (17) | 21 (24) | 27 (16) | 8 (10) |
| Hypokalemia | 29 (17) | 21 (19) | 23(14) | 18 (17) | 24 (14) | 12 (14) | 22 (13) | 14 (18) |
| Hypoalbuminemia | 26 (15) | 17 (15) | 11 (6) | 6 (5.6) | 38 (23) | 19 (22) | 24 (14) | 11 (14) |
Source: FDA Analysis
Abbreviations: TEAE, treatment-emergent adverse event
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION