Drug Trials Snapshots: XENOVIEW
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the XENOVIEW Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
XENOVIEW (xenon Xe 129 hyperpolarized)
Zee-non Xe 129 hye-per-POE-la-ryzd
Polarean, Inc.
Approval date: December 23, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XENOVIEW is a hyperpolarized contrast agent that is indicated for use with magnetic resonance imaging (MRI) for evaluation of lung ventilation in adults and pediatric patients aged 12 years and older. Limitations of Use: XENOVIEW has not been evaluated for use with lung perfusion imaging.
How is this drug used?
XENOVIEW is administered by a healthcare provider by inhalation immediately before imaging.
Who participated in the clinical trials?
The FDA approved XENOVIEW based on evidence from two clinical trials in 83 patients with various lung disorders who were being evaluated for possible lung resection or lung transplantation. The trials were conducted at five sites in the United States and assessed both efficacy and safety of XENOVIEW.
How were the trials designed?
XENOVIEW was evaluated in two clinical trials of 83 adult patients with pulmonary disorders who each underwent sequential lung ventilation imaging with XENOVIEW with MRI and an approved comparator, Xe 133 scintigraphy.
In Study 1, patients were imaged to help plan possible lung resection. To determine the benefit of XENOVIEW, estimates of the percentage of lung ventilation predicted to remain after surgery made with XENOVIEW with MRI and comparator imaging were evaluated for equivalence.
In Study 2, patients were imaged to help plan possible lung transplantation. To determine the benefit of XENOVIEW, estimates of the percentage of lung ventilation contributed by the right lung made with XENOVIEW with MRI and comparator imaging were evaluated for equivalence.
How were the trials designed?
The efficacy and safety of XENOVIEW were evaluated in two prospective, multi-center, open-label, cross-over clinical trials that compared XENOVIEW with MRI to an approved ventilation imaging comparator, xenon Xe 133 scintigraphy, in adult patients with pulmonary disorders. Each patient sequentially underwent XENOVIEW with MRI and comparator imaging in randomized order. A mean dose equivalent of 99 mL of hyperpolarized xenon 129 was administered in a mean volume of 330 mL xenon gas in these trials.
In Study 1, patients were imaged for planning of possible lung resection, and mean within-patient difference in the predicted post-operative percentage of remaining lung ventilation between XENOVIEW with MRI and comparator imaging was the primary efficacy endpoint. In Study 2, patients were imaged for planning of possible lung transplantation. Mean within-patient difference in the percentage of overall lung ventilation contributed by the right lung between XENOVIEW with MRI and comparator imaging was the primary endpoint. In both studies, the primary efficacy endpoint was compared to a prespecified equivalence interval of -14.7% to +14.7%.
DEMOGRAPHIICS SNAPSHOT
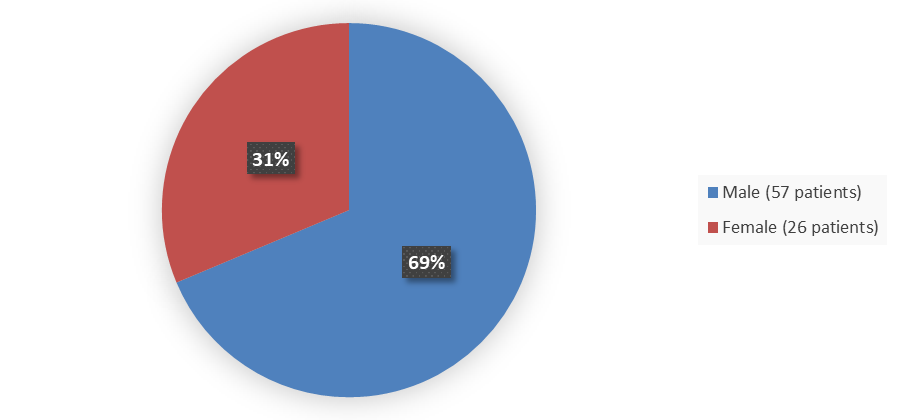
Figure 1. Baseline Demographics by Sex in the Safety Population
Source: Adapted from FDA Review
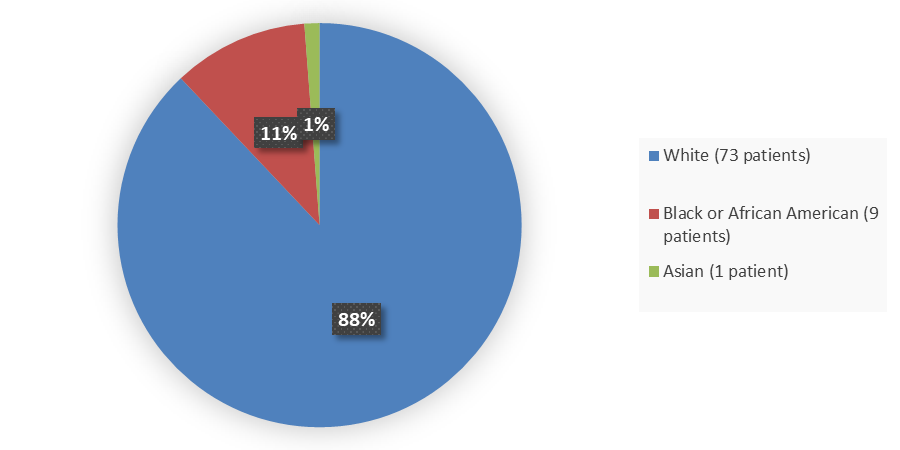
Figure 2. Baseline Demographics by Race in the Safety Population
Source: Adapted from FDA Review
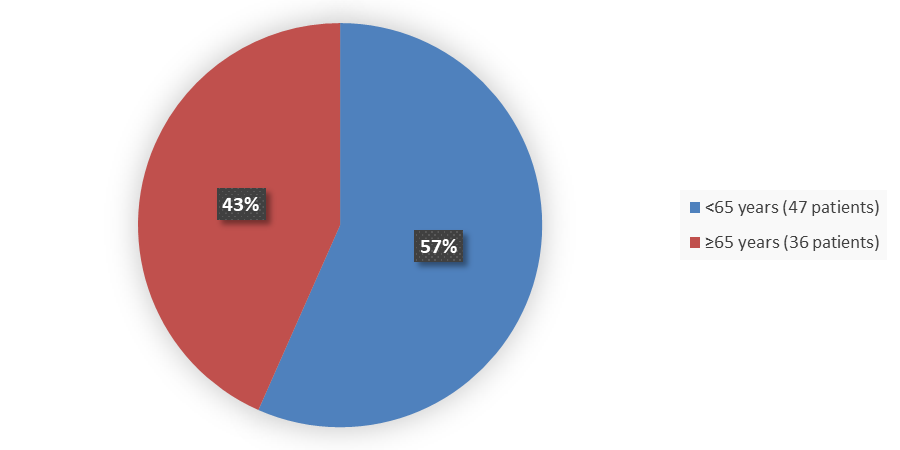
Figure 3. Baseline Demographics by Age in the Safety Population
Source: Adapted from FDA Review
Figure 4. Baseline Demographics by Ethnicity in the Safety Population
Source: Adapted from FDA Review
Who participated in the trials?
The FDA approved XENOVIEW based on evidence from two clinical trials. Demographics of the safety population are presented in Table 1.
Table 1. Demographics
| Demographic Parameters | Study 1 N=34 |
Study 2 |
Total N=83 |
| Sex, n (%) | |||
| Female | 11 (32) | 15 (31) | 26 (31) |
| >Male | >23 (68) | 34 (69) | 57 (69) |
| Age | |||
| Mean years (SD) | 62.2 (9.7) | 61.5 (10.5) | 61.8 (10.2) |
| Min, max years | 25, 77 | 19, 77 | 19, 77 |
| Race, n (%) | |||
| Asian | 1 (3) | 0 (0) | 1 (1) |
| Black or African American | 6 (18) | 3 (6) | 9 (11) |
| White | 27 (79) | 46 (94) | 73 (88) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 0 | 1 (2) | 1 (1) |
| Not Hispanic or Latino | 34 (100) | 48 (98) | 82 (99) |
Source: Adapted from FDA Review
What are the benefits of this drug?
XENOVIEW with MRI allows evaluation of lung ventilation, which can be used for planning lung surgery and in other clinical contexts.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy of XENOVIEW with MRI was evaluated for equivalence to an approved comparator, Xe 133 scintigraphy, in adult patients undergoing pre-operative evaluation for certain lung surgeries.
In Study 1 (N=31 patients), the mean within-patient difference in the predicted post-operative percentage of remaining lung ventilation between XENOVIEW with MRI and comparator imaging was within a pre-specified equivalence interval with an observed estimate of 1.4% (95% confidence interval: -0.8%, 3.6%). In Study 2 (N=49 patients), the mean within-patient difference in the percentage of overall lung ventilation contributed by the right lung between XENOVIEW with MRI and comparator imaging was within a pre-specified equivalence interval with an observed estimate of -1.6% (95% confidence interval: -3.7%, 0.5%).
The effectiveness of XENOVIEW with MRI in adults was extrapolated to pediatric patients aged 12 years and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: XENOVIEW with MRI worked similarly to a comparator in both female and male patients.
- Race: XENOVIEW with MRI worked similarly to a comparator in both White and black participants in one clinical trial. In another clinical trial, the number of patients of races other than White was small; therefore, differences in how XENOVIEW with MRI worked relative to a comparator among races could not be determined.
- Age: XENOVIEW with MRI worked similarly to a comparator in both patients younger than 65 years of age and patients 65 years of age and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
In Study 1, equivalence of XENOVIEW with MRI and comparator imaging was demonstrated in patients younger than 65 years of age (95% CI: -1.72, 3.82) and those 65 years of age and older (95% CI: -1.73, 6.42), in male (95% CI: -0.39, 2.77) and female (95% CI: -4.86, 8.69) patients, and in White patients (95% CI: -1.39, 3.72) and patients of other races (Black and Asian) (95% CI: -3.06, 7.72).
In Study 2, equivalence of XENOVIEW with MRI and comparator imaging was demonstrated in patients younger than 65 years of age (95% CI: -2.64, 4.57) and those 65 years of age and older (95% CI: -6.05, -2.05) and in both male (95% CI: -4.55, -0.93) and female (95% CI: -4.74, 6.75) patients. Equivalence of the two imaging methods was demonstrated in White patients (95% CI: -3.49, 0.89). However, there were too few patients of races other than White (N=3) to know whether there were any differences in how well XENOVIEW with MRI worked across races.
What are the possible side effects?
Inhalation of XENOVIEW may cause temporary decrease in blood oxygen levels in susceptible patients. The most common side effects of XENOVIEW include mouth and throat pain, headache, and dizziness.
What are the possible side effects (results of trials used to assess safety)?
In the 83 adult patients with various lung disorders who received XENOVIEW in efficacy trials, adverse reactions reported in more than one patient were oropharyngeal pain (N=4 patients), headache (N=2 patients), and dizziness (N=2 patients).
In pooled data of 123 adult patients from published literature, most of whom had lung disorders, the following transient adverse reactions were reported with hyperpolarized xenon Xe 129: numbness, tingling, euphoria, and dizziness. Overall frequency of these adverse reactions could not be determined from the published information.
In pooled data of 120 pediatric patients aged 6 to 18 years from published literature, most of whom had lung disorders, the following transient adverse reactions were reported: blood oxygen desaturation, heart rate elevation, numbness, tingling, dizziness, and euphoria. Overall frequency of these adverse reactions could not be determined from the published information.
Were there any differences in side effects among sex, race, and age?
- Sex: The observed rates of adverse events were similar among males and females.
- Race: The observed rates of adverse events were similar among White participants and Black or African American participants. The numbers of other races were too small to make comparisons.
- Age: There was no observed relationship between age and the occurrence of an adverse event.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
In efficacy trials, 15 patients (18%) reported adverse events.
Of patients reporting adverse events, the mean (SD) age was 63.5 (7.2) years. The mean (SD) of the overall efficacy trial population was similar at 61.8 (10.1) years, suggesting that age does not significantly influence the occurrence of adverse events.
Adverse events were reported by 11 males and 4 females which represent 73% and 27% of patients reporting adverse events, respectively. Males represented 69% of the efficacy trial population and females represented 31%, suggesting that sex does not significantly influence the occurrence of adverse events.
Of patients reporting adverse events, 87% were White and 13% were Black. The efficacy trial population included 88% White patients and 11% Black patients, suggesting that adverse events do not occur disproportionately in White and Black patients.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.