Drug Trials Snapshots: ZYNYZ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the ZYNYZ Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
ZYNYZ (retifanlimab-dlwr)
ZYE-niz
Incyte Corporation
Approval date: March 22, 2023
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ZYNYZ is used to treat a type of skin cancer called Merkel cell carcinoma (MCC) in adults. It is used in patients where the cancer has spread through the body (metastatic) or cannot be controlled with surgery and/or radiation.
How is this drug used?
ZYNYZ is given by a healthcare provider using a needle placed in a vein (known as intravenous infusion) over 30 minutes. ZYNYZ is given every four weeks.
Who participated in the clinical trials?
The FDA approved ZYNYZ based on evidence from one clinical trial of patients with metastatic or recurrent locally advanced MCC who had not received prior chemotherapy. A total of 65 patients were evaluated to see if the size of their tumor changed (efficacy population) and 105 patients were evaluated for side effects (safety population). The trial was conducted at 34 sites in Italy, France, the United States, Poland, Canada, Switzerland, Hungary, the Czech Republic, Germany, Spain, and the United Kingdom.
How were the trials designed?
The benefit and side effects of ZYNYZ were evaluated in one clinical trial of patients with metastatic or recurrent locally advanced MCC. The trial measured the percentage of patients who experienced partial shrinkage or complete disappearance of their tumor. All patients received ZYNYZ once every four weeks until either the tumors grew or the patients developed an unacceptable side effect.
The benefit of ZYNYZ was evaluated by the percentage of patients who achieved partial or complete shrinkage of their tumors (objective response rate or ORR) and by how long that shrinkage lasted (duration of response or DOR).
DEMOGRAPHICS SNAPSHOT
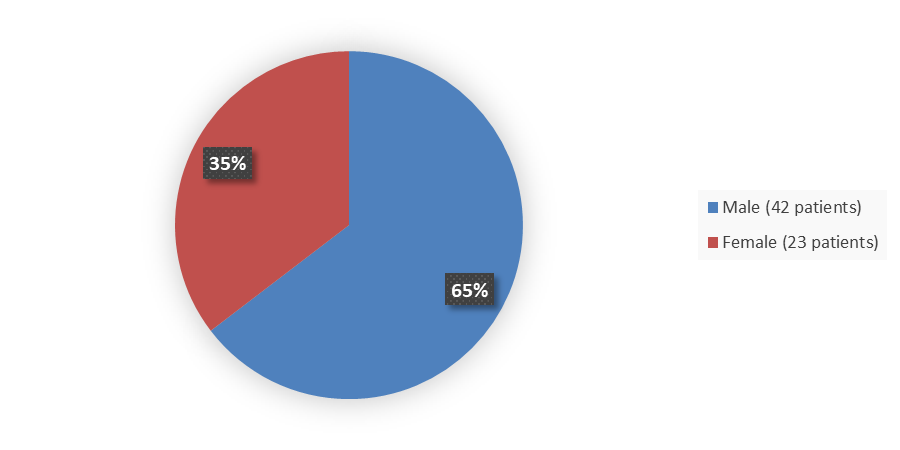
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy of ZYNYZ.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
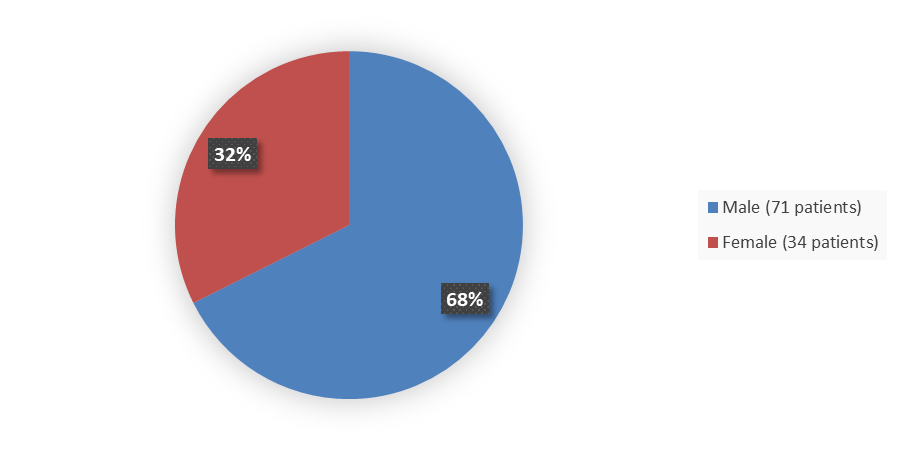
Figure 2 summarizes how many patients by sex were enrolled in the trial used to evaluate the side effects of ZYNYZ.
Figure 2. Baseline Demographics by Sex, Safety Population
Source: Adapted from FDA Review
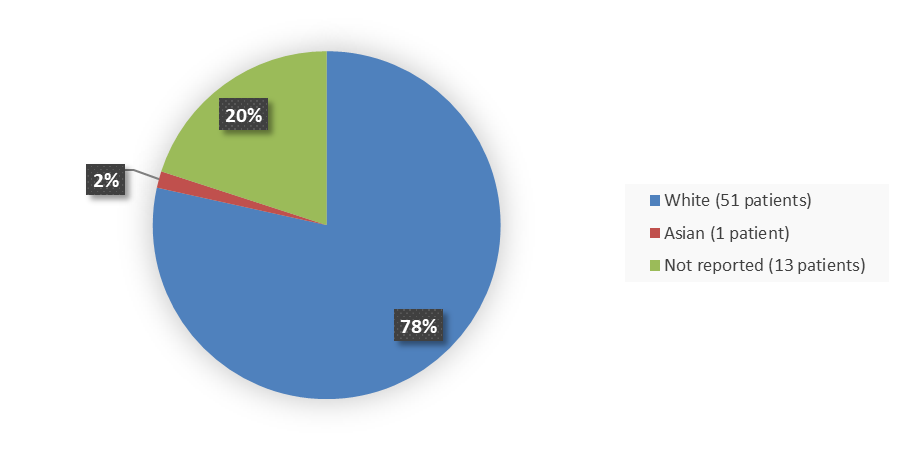
Figure 3 summarizes how many patients by race enrolled in the clinical trial used to evaluate the efficacy of ZYNYZ.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
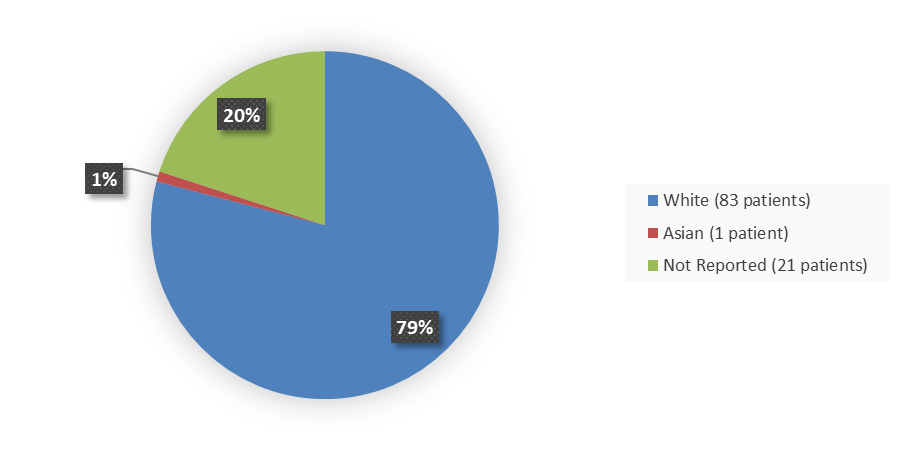
Figure 4 summarizes how many patients by race were enrolled in the trial used to evaluate the side effects of ZYNYZ.
Figure 4. Baseline Demographics by Race, Safety Population
Source: Adapted from FDA Review
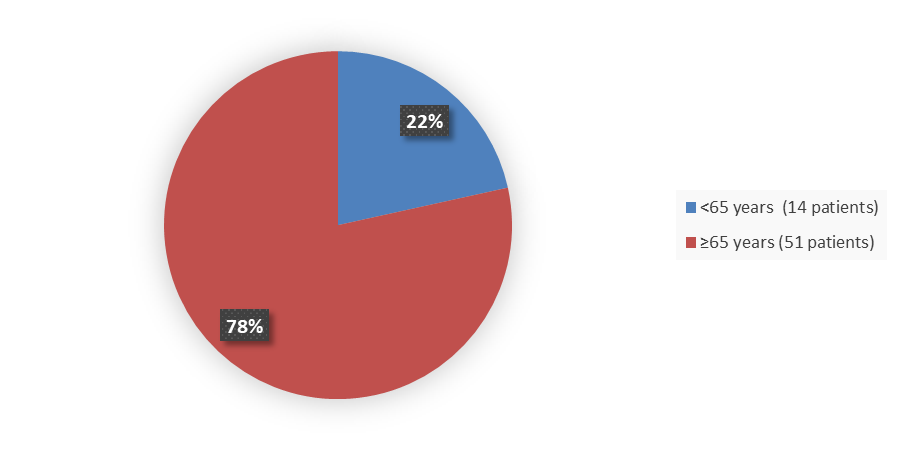
Figure 5 summarizes how many patients by age were enrolled in the clinical trial used to evaluate the efficacy of ZYNYZ.
Figure 5. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
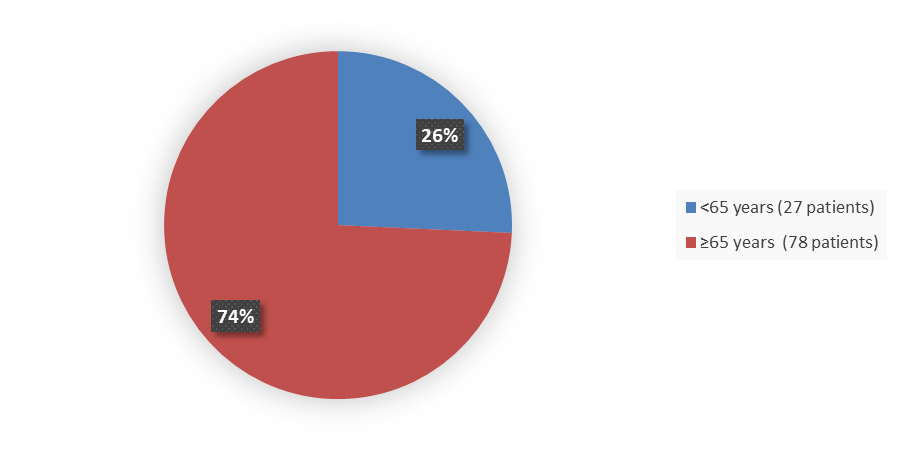
Figure 6 summarizes how many patients by age were in the trial used to evaluate the side effects of ZYNYZ.
Figure 6. Baseline Demographics by Age, Safety Population
Source: Adapted from FDA Review
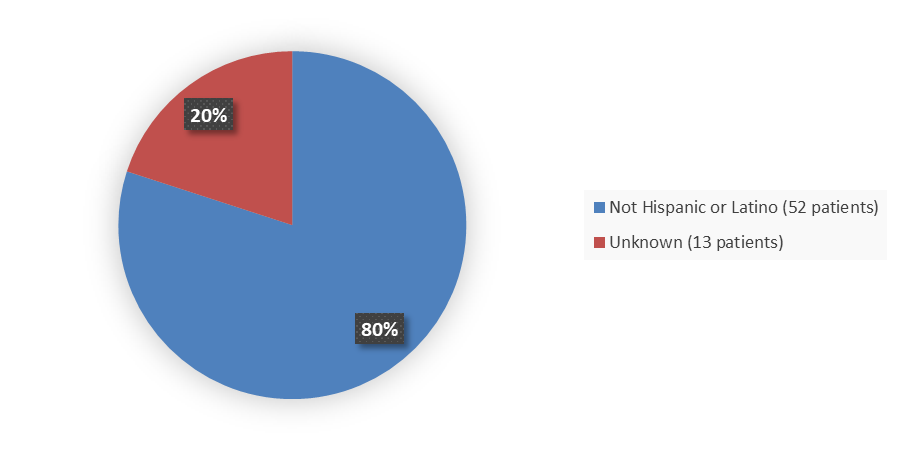
Figure 7 summarizes how many patients by ethnicity were enrolled in the trial used to evaluate the efficacy of ZYNYZ.
Figure 7. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Demographics Table, Efficacy Population
|
Variable |
Chemotherapy-Naïve MCC N=65 |
|
Age, years |
|
|
Mean (SD) |
71.4 (10.26) |
|
Median (minimum, maximum) |
71.0 (44, 90) |
|
Age group, years, n (%) |
|
|
<65 |
14 (21.5) |
|
≥65 |
51 (78.5) |
|
Sex, n (%) |
|
|
Male |
42 (64.6) |
|
Female |
23 (35.4) |
|
Race, n (%) |
|
|
White/Caucasian |
51 (78.5) |
|
Asian |
1 (1.5) |
|
Othera |
13 (20.0) |
|
Ethnicity, n (%) |
|
|
Not Hispanic or Latino |
52 (80.0) |
|
Not reported |
13 (20.0) |
Source: Adapted from FDA Review
a Other includes participants from France, where information on race is not collected per regulatory requirements.
Abbreviations: MCC, Merkel cell carcinoma; SD, standard deviation
What are the benefits of this drug?
In the trial PODIUM-201, 34 of 65 patients (52%) with MCC who were treated with ZYNYZ experienced a decrease in the size of their tumors (34%) or their tumors could no longer be detected (18%). Tumor shrinkage lasted more than 6 months for 76% of those patients and more than 12 months for 62% of patients.
ZYNYZ was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes efficacy results for the clinical trial based on ORR and DOR according to Response Evaluation Criteria in Solid Tumors v1.1 as measured by Independent Central Review.
Table 2. Efficacy Results
|
Efficacy Parameter |
ZYNYZ |
|
Objective response rate % (95% CI)* |
52 (40, 65) |
|
Complete responses, n (%) |
12 (18) |
|
Partial responses, n (%) |
22 (34) |
|
Duration of response |
N=34 |
|
Range, months |
1.1 to 24.9+ |
|
Patients with DOR ≥6 months, n (%) |
26 (76) |
|
Patients with DOR ≥12 months, n (%) |
21 (62) |
Source: Adapted from ZYNYZ Prescribing Information
* 95% exact confidence interval using Clopper-Pearson method
Abbreviations: CI, confidence interval; DOR, duration of response; +, denotes ongoing response
Were there any differences in how well the drug worked in clinical trials among sex, race, and age ?
- Sex: The observed effect of ZYNYZ was similar for females and males.
- Race: The number of patients of races other than White was small; therefore, differences in how ZYNYZ worked among races could not be determined.
- Age: The number of patients of <65 years was small; therefore, differences in how ZYNYZ worked among different age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes efficacy results by sex. Differences in how well ZYNYZ works among age and race could not be determined in the trial.
Results should be interpreted with caution given the small sample size overall and the limited number of patients in each subgroup.
Table 3. Subgroup Analysis of Objective Response
|
Subgroup |
Number of Patients |
ORR % (95% CI) |
|
Male |
42 |
52 (36, 68) |
|
Female |
23 |
52 (31, 73) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; ORR, objective response rate
What are the possible side effects?
ZYNYZ can cause serious immune reactions including inflammation of the lungs, gut, liver, kidneys, hormonal glands and skin, as well as infusion-related reactions.
The most common side effects are tiredness, muscle and bone pain, itchy skin, diarrhea, skin rash, fever, and nausea.
What are the possible side effects (results of trials used to assess safety)?
Table 4 below summarizes the most common side effects that occurred in at least 10% of the patients in the safety population (N=105).
Table 4. Side Effects in ≥10% of Patients, Safety Population
|
Side Effect |
ZYNYZ, N=105 |
|
|
All Grades (%) |
Grades 3 to 4 (%) |
|
|
Fatiguea |
28 |
1 |
|
Musculoskeletal painb |
22 |
2.9 |
|
Itchy skin |
18 |
0 |
|
Diarrhea |
15 |
0 |
|
Rashc |
11 |
1 |
|
Diarrhea |
15 |
0 |
|
Fever |
10 |
0 |
|
Nausea |
10 |
0 |
Source: ZYNYZ Prescribing Information
Graded according to NCI CTCAE v5.0.
a Includes fatigue and asthenia.
b Includes arthralgia, back pain, bone pain, pain in extremity, neck pain, and myalgia.
c Includes rash, dermatitis, dermatitis bullous, rash erythematous, rash maculo-papular, rash papular, and rash pruritic.
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar for females and males.
- Race: The number of patients of races other than White was small; therefore, differences in how ZYNYZ worked among races could not be determined.
- Age: The number of patients of <65 years was small; therefore, differences in how ZYNYZ worked among different age groups could not be determined.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION