Drug Trials Snapshots: CABLIVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the CABLIVI Package Insert for complete information.
CABLIVI (caplacizumab-yhdp)
cab-LIV-ee
Ablynx N.V.
Approval date: February 6, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CABLIVI is a drug used to treat adults with an episode of acquired thrombotic thrombocytopenic purpura (aTTP).
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare, life-threatening blood clotting disorder. During an episode of aTTP, clots form throughout the body and lead to low platelets count, destroy red blood cells, and can damage organs (especially the brain and heart).
How is this drug used?
CABLIVI is used in combination with standard treatment of plasma exchange and medications that suppresses the immune system.
- The first dose of CABLIVI is injected into a vein (intravenous) by a healthcare provider at least 15 minutes before the first plasma exchange.
- Treatment is continued with an injection under the skin (subcutaneous) after daily plasma exchange and for 30 days after daily plasma exchange is stopped.
What are the benefits of this drug?
In patients with aTTP who required plasma exchange to treat their condition, platelet count returned to the normal range more quickly in patients treated with CABLIVI than in patients treated with placebo. Patients treated with CABLIVI also had fewer deaths, repeat episodes of aTTP and major blood clotting episodes when compared to patients that received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The figure below summarizes efficacy results for the evaluated patients in Trial 1. The primary outcome was the time to platelet count response defined as a platelet count > 150,000/µL followed by cessation of daily plasma exchange within 5 days.
Figure 4. Platelet Response over Time (Trial 1)
CABLIVI Prescribing Information
The table below summarizes secondary efficacy results for the evaluated patients in Trial 1.
Table 2. Number of Patients in Trial 1 with aTTP-Related Death, a Recurrence of aTTP, or at Least One Treatment-Emergent Major Thromboembolic Event (ITT Population)
| CABLIVI N=72 |
Placebo N=73 |
|
|---|---|---|
| Number of patients with | n (%)* | n (%) |
| TTP-related death | 0 | 3 (4.1) |
| Recurrence of TTP (exacerbation)† | 3 (4.2) | 28 (38.4) |
| At least one treatment-emergent major thromboembolic event | 6 (8.5) | 6 (8.2) |
| Total‡ | 9 (12.7) | 36 (49.3) |
N = number of patients within the population of interest (by treatment group); n = number of patients with events;
TTP = thrombotic thrombocytopenic purpura; ITT = intent to treat; * based on 71 patients who received at least one dose of study drug.
† Exacerbation defined as thrombocytopenia after initial recovery of platelet count (platelet count ≥150,000/µL with subsequent stop of daily plasma exchange within 5 days) that required reinitiation of daily plasma exchange during the 30-day post daily plasma exchange period.
‡ p <0.0001
CABLIVI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: CABLIVI works similarly in both men and women.
- Race: The majority of patients were White. The number of patients in other races were limited; therefore, differences in how CABLIVI worked among races could not be determined.
- Age: CABLIVI works similarly in patients younger and older than 50 years of age. The majority of patients were less than 65 years of age; therefore, differences in how CABLIVI worked among patients older than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex, and age based on the time to platelet count response.
Table 2. Hazard Ratios for Time to Platelet Response by Sex, Race, and Age
| Subgroup | CABLIVI | Placebo | HR | CI |
|---|---|---|---|---|
| Overall | 66/5/71 | 66/7/73 | 1.58 | 1.113, 2.242 |
| Sex | ||||
| Men | 20/2/22 | 21/1/22 | 1.89 | 0.9924, 3.587 |
| Women | 46/3/49 | 45/6/51 | 1.46 | 0.9623, 2.205 |
| Race | ||||
| White | 43/3/46 | 47/3/50 | 1.35 | 0.8855, 2.055 |
| Other | 23/2/25 | 19/4/23 | 2.24 | 1.197, 4.181 |
| Age Group (years) | ||||
| < 50 | 46/2/48 | 44/2/46 | 1.91 | 1.246, 2.922 |
| > 50 | 20/3/23 | 22/5/27 | 1.14 | 0.6167, 2.121 |
FDA Review
What are the possible side effects?
CABLIVI may cause serious side effects such as increased bleeding.
The most common side effects are nosebleeds, headache, and bleeding gums.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in patients with aTTP in the clinical trials (safety population).
Table 3. Adverse Reactions in ≥2% of Patients Treated with CABLIVI and More Frequent than Placebo during the Blinded Periods of aTTP Studies (Trial 1 and Trial 2)
| Adverse Reaction by Body System | CABLIVI (N=106) n (%) |
Placebo (N=110) n (%) |
|---|---|---|
| Gastrointestinal disorders | ||
| Gingival bleeding | 17 (16) | 3 (3) |
| Rectal hemorrhage | 4 (4) | 0 (0) |
| Abdominal wall hematoma | 3 (3) | 1 (1) |
| General disorders and administration site conditions | ||
| Fatigue | 16 (15) | 10 (9) |
| Pyrexia | 14 (13) | 12 (11) |
| Injection site hemorrhage | 6 (6) | 1 (1) |
| Catheter site hemorrhage | 6 (6) | 5 (5) |
| Injection site pruritus | 3 (3) | 0 (0) |
| Musculoskeletal and connective tissue disorders | ||
| Back Pain | 7 (7) | 4 (4) |
| Myalgia | 6 (6) | 2 (2) |
| Nervous system disorders | ||
| Headache | 22 (21) | 15 (14) |
| Paresthesia | 13 (12) | 11 (10) |
| Renal and urinary disorders | ||
| Urinary tract infection | 6 (6) | 4 (4) |
| Hematuria | 4 (4) | 3 (3) |
| Reproductive system and breast disorders | ||
| Vaginal hemorrhage | 5 (5) | 2 (2) |
| Menorrhagia | 4 (4) | 1 (1) |
| Respiratory, thoracic and mediastinal disorders | ||
| Epistaxis | 31 (29) | 6 (6) |
| Dyspnea | 10 (9) | 5 (5) |
| Skin and subcutaneous tissue disorders | ||
| Urticaria | 15 (14) | 7 (6) |
CABLIVI Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among men and women.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in side effects among races could not be determined.
- Age: The majority of patients were less than 65 years of age; therefore, differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the incidence of bleeding by subgroup.
Table 4. Incidence of Adverse Events with Bleeding by Age, Sex, and Race
| Demographic Characteristic | CABLIVI n/N (%) |
Placebo n/N (%) |
|---|---|---|
| Sex | ||
| Men | 19/33 (58) | 17/41 (41) |
| Women | 43/73 (59) | 30/69 (43) |
| Race | ||
| White | 45/77 (58) | 37/83 (45) |
| Other* | 16/29 (55) | 4/27 (15) |
| Age Group | ||
| Less than 65 years | 60/101 (59) | 44/101 (44) |
| 65 years and older | 2/5 (40) | 3/9 (33) |
*Other includes Black or African American, Asian, Native Hawaiian or Other Pacific Islander
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved CABLIVI based on evidence from two clinical trials (Trial 1/NCT02553317 and Trial 2/NCT01151423) of 216 adults with aTTP. The trials were conducted in Asia, Canada, Europe, Great Britain and the United States.
Demographics of the patients who provided data for evaluation of benefits (efficacy population) are presented in Table 6, under the MORE INFO section.
The population that provided data for side effects of CABLIVI (safety population) is presented below.
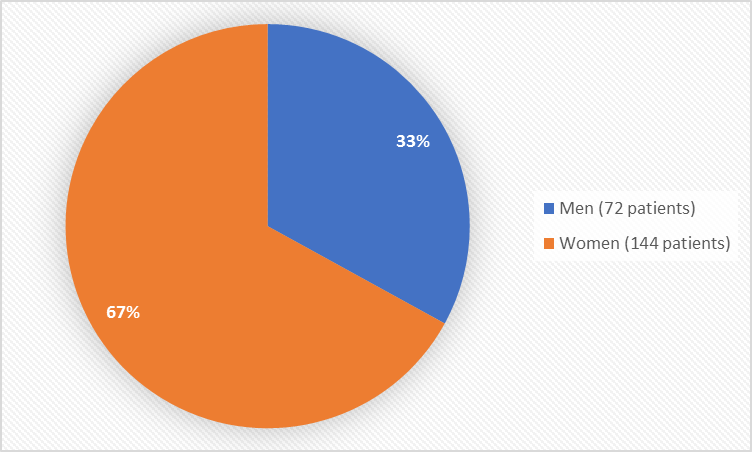
Figure 1 summarizes how many men and women were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
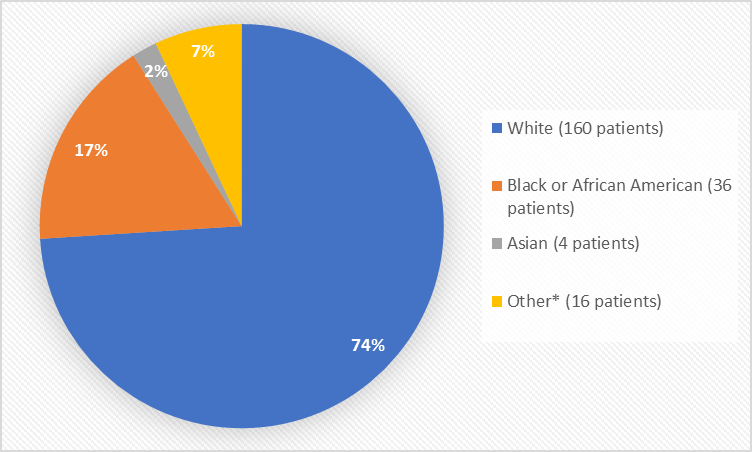
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race
Table 1. Demographics of Safety Trials by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 160 | 74% |
| Black or African American | 36 | 17% |
| Asian | 4 | 2% |
| Native Hawaiian or Other Pacific Islander | 1 | 1% |
| Other | 15 | 6% |
Clinical Trial Data
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The tables below summarize demographics of the safety and efficacy populations.
Table 5. Demographic Characteristics of the Safety Population
| Characteristic | CABLIVI (n=106) n (%) |
Placebo (n=110) n (%) |
Total (n=216) n (%) |
|---|---|---|---|
| Sex | |||
| Men | 33 (31.1%) | 39 (35.5%) | 72 (33.3%) |
| Women | 73 (68.9%) | 71 (64.5%) | 144 (66.7%) |
| Race | |||
| White | 77 (72.6%) | 83 (75.5%) | 160 (74.1%) |
| Black/African | 19 (17.9%) | 17(15.5%) | 36 (16.7%) |
| Asian | 4 (3.8%) | 0 (0%) | 4 (1.8%) |
| Native Hawaiian or Other Pacific Islander | 1 (0.9%) | 0 (0%) | 1 (0.5%) |
| Other | 5 (4.7%) | 10 (9.1%) | 15 (6.9%) |
| Age (years) | |||
| Mean (SD) | 43.5 (13.2) | 45.9 (13.9) | 44.7 (13.6) |
| Median Age | 44 (18, 77) | 45 (21, 79) | 44 (18, 79) |
| Age Group | |||
| < 50 years | 75 (70.8%) | 72 (65.5) | 147 (68.1%) |
| > 50 years | 31 (29.2%) | 38 (34.5%) | 69 (31.9%) |
| < 65 years | 101 (95.3%) | 101 (91.8%) | 202 (93.5%) |
| > 65 years | 5 (4.7%) | 9 (8.2%) | 14 (6.5%) |
| Ethnicity | |||
| Hispanic | 4 (3.7%) | 2 (1.8%) | 6 (2.8%) |
| Non-Hispanic | 102 (96.2%) | 108 (98.2%) | 210 (97.2%) |
| Region | |||
| Asia | 8 (7.5%) | 9 (8.2%) | 17 (7.9%) |
| Canada | 7 (6.6%) | 6 (5.4%) | 13 (6.0%) |
| Europe | 71 (67.0%) | 64 (58.2%) | 135 (62.5%) |
| Rest of the World | 2 (1.9%) | 2 (1.8%) | 4 (1.8%) |
| United States | 18 (17.0%) | 29 (26.4) | 47 (21.8%) |
Clinical Trial Data
Table 6. Demographic Characteristics of the Intent-to-Treat (ITT) Population
| Characteristic | CABLIVI (n=72) |
Placebo (n=73) |
Total (n=145) |
|---|---|---|---|
| Sex | |||
| Men | 23 (31.9%) | 22 (30.1%) | 45 (31%) |
| Women | 49 (68.1%) | 51 (69.9%) | 100 (69%) |
| Race | |||
| White | 47 (65.3%) | 50 (68.5%) | 97 (66.9%) |
| Black/African | 15 (20.8%) | 13 (17.8%) | 28 (19.3%) |
| Asian | 4 (5.6%) | 0 (0%) | 4 (2.8%) |
| Native Hawaiian or Other Pacific Islander | 1 (1.4%) | 0 (0%) | 1 (0.7%) |
| Other | 5 (6.9%) | 10 (13.7) | 15 (10.3%) |
| Age Group (years) | |||
| < 50 years | 48 (66.7%) | 46 (63.0%) | 94 (64.8%) |
| > 50 years | 24 (33.3%) | 27 (37.0%) | 51 (35.2%) |
| Ethnicity | |||
| Hispanic | 4 (5.6%) | 2 (2.7%) | 6 (4.1%) |
| Non-Hispanic | 68 (94.4%) | 71 (97.3%) | 139 (95.9%) |
| Region | |||
| Europe | 46 (63.9%) | 36 (49.3%) | 82 (56.6%) |
| Rest of the World | 9 (12.5%) | 9 (12.3%) | 18 (12.4%) |
| North America | 17 (23.6%) | 28 (38.4%) | 45 (31%) |
Clinical Trial Data
How were the trials designed?
The benefit and side effects of CABLIVI were evaluated in two clinical trials.
Trial 1 enrolled adults 18 to 79 years old who had an episode of aTTP. Patients in both groups received standard of care treatment with plasma exchange and immunosuppressive therapy. Patients were randomized to receive CABLIVI or placebo daily in addition to plasma exchange and for 30 days after their last plasma exchange.
The benefit of CABLIVI was assessed by measuring the time needed for a patient’s platelet count to return to normal and comparing it with patients treated with placebo. The trial also evaluated the number of patients who died due to aTTP, had a second episode of aTTP, or had at least one major blood clotting event.
Trial 2 had a similar design as Trial 1, but healthcare providers knew which treatment was given before the trial was completed. The data were used primarily to assess side effects.
How were the trials designed?
The efficacy and safety of CABLIVI were evaluated in two clinical trials.
Trial 1 was a randomized, placebo controlled, double blind trial evaluating CABLIVI in addition to standard of care treatment with plasma exchange and immunosuppression therapy for the treatment of aTTP in adult patients. Patients were randomized to receive a single 11 mg bolus intravenous injection or placebo prior to the first plasma exchange, followed by a daily subcutaneous injection of 11 mg CABLIVI or placebo after completion of plasma exchange and for 30 days thereafter. Treatment could be extended weekly for a maximum of 4 weeks. The primary efficacy endpoint was time to platelet response, defined by platelet count > 15,000/µL followed by cessation of daily plasma exchange within 5 days. Secondary endpoints evaluated the number of patients who died due to aTTP, had a recurrence of aTTP, or had at least one major thromboembolic event.
Trial 2 was a randomized, placebo controlled, single blind trial with a similar design as Trial 1. The data were used to primarily assess side effects.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.