Homeopathic Products
Visit some homeopathic products may put you at risk for more information
What is homeopathy?
Homeopathy is an alternative medical practice that was developed in the late 1700s. Homeopathy is generally based on two main principles:
- that a substance that causes symptoms in a healthy person can be used in diluted form to treat symptoms and illnesses, a principle known as “like-cures-like”; and
- the more diluted the substance, the more potent it is, which is known as the “law of infinitesimals.”
Historically, homeopathic products have been identified through “provings,” in which substances are administered to healthy individuals in concentrations that cause symptoms. Symptoms experienced by individuals are recorded to indicate possible therapeutic uses for the substances. In other words, if a substance causes a particular symptom, individuals experiencing that symptom would be treated with a diluted solution made from that substance.
Homeopathic products are labeled as containing a wide range of substances that are highly diluted. These substances can include ingredients derived from plants, healthy or diseased animal or human sources, minerals and chemicals. They can also include known poisons or toxins. Examples of these substances include:
- nux vomica (the source of strychnine)
- belladonna (deadly nightshade)
- mercurius solubilis (mercury)
- plumbum aceticum (lead)
How to identify a homeopathic product
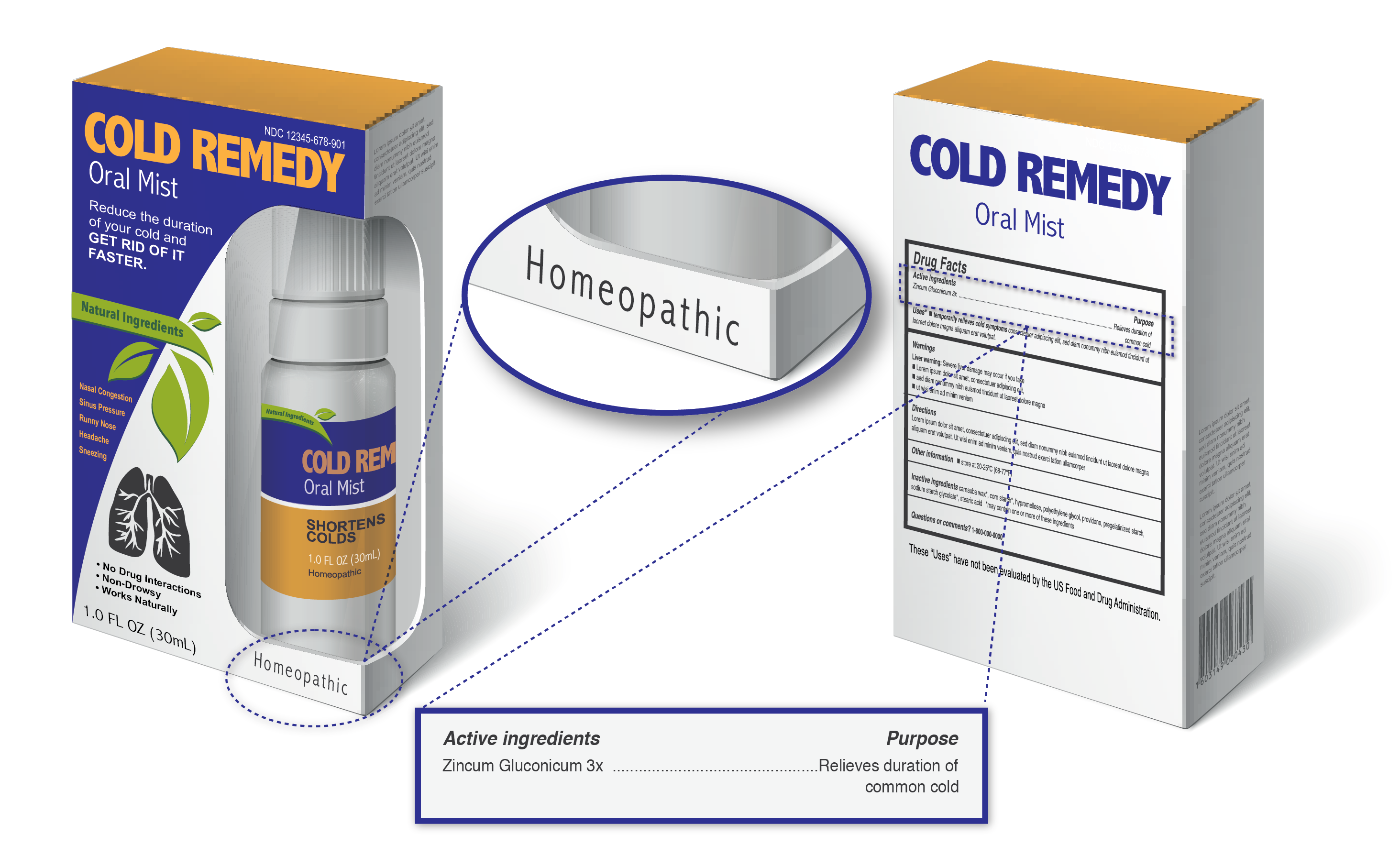
Homeopathic products are often marketed as natural, safe and effective alternatives to FDA-approved prescription and nonprescription products but it is important to consider that homeopathic products marketed in the U.S. have not been reviewed by the FDA for safety and effectiveness. Additionally, there have been multiple situations in which homeopathic products have posed a significant risk to patients in recent years. Homeopathic products are often placed on the store shelves next to nonprescription (over-the-counter) drugs which have been reviewed by the FDA for safety and effectiveness which may make it difficult for consumers to identify the products as homeopathic.
The best way to identify a homeopathic product is by looking for specific information on the label. Homeopathic products generally include:
- The word “Homeopathic”
- Ingredients often listed in the Latin or scientific names of their sources instead of their common names
- The ingredients listed in terms of dilution, such as 1X, 6X, 2C
FDA’s concerns about product safety
There have been several instances of serious harm to consumers caused by homeopathic products in recent years. While homeopathic products are generally labeled as highly diluted, some of these products have been found to contain measurable amounts of active ingredients. Because homeopathic products are sometimes made from known poisons or toxins, containing measurable amounts of such ingredients could cause significant patient harm.
Improper manufacturing of homeopathic products also can increase the potential for contamination and can cause incorrect dilutions. Additionally, consumers may use unapproved homeopathic drugs instead of safe and effective FDA-approved treatments which may result in delaying or stopping effective treatment of their condition or disease.
Visit our page on homeopathic products that may put you at risk for more information.
No homeopathic product is FDA-approved
There are no FDA-approved products labeled as homeopathic. Homeopathic products marketed in the U.S. have not been reviewed by the FDA for safety and effectiveness to diagnose, treat, cure or prevent any diseases or conditions. FDA’s evidence-based drug review process plays an essential role in ensuring drugs are safe, effective for their intended uses and made with quality manufacturing processes.
Products that have not been evaluated for safety, effectiveness or quality may harm consumers who choose to use them.
Since 2020, the FDA has issued more than 20 warning letters to companies for violations of federal law, including serious quality violations such as sterility concerns and contamination.
FDA recommends consumers talk to their doctor
FDA recommends talking to your doctor or pharmacist about which products are safe and effective for you.
Visit “What Does FDA Approve? Part 2” for more information.
Regulating homeopathic products
Under federal law, homeopathic products are subject to the same requirements related to approval, adulteration and misbranding as other drugs. Since homeopathic products have not been approved by FDA for any use, they may not meet modern standards for safety, effectiveness and quality.
On December 6, 2022, FDA issued a final guidance, Homeopathic Drug Products, that describes the agency’s risk-based enforcement approach to homeopathic products marketed without FDA approval. Specifically, FDA intends to focus its enforcement authorities on products:
- with reports of injury or that raise potential safety concerns;
- that contain or purport to contain ingredients associated with potentially significant safety concerns;
- for routes of administration other than oral or topical, such as products for use in the eye or taken nasally or by injection;
- that claim to treat or prevent serious and/or life-threatening diseases and conditions, such as cancer;
- marketed to vulnerable populations, including children, pregnant women and the elderly; or
- with significant quality issues.