Drug Trials Snapshots: DARZALEX
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the DARZALEX Prescribing Information
DARZALEX (daratumumab)
(Dar'-zah-lex)
Jannsen Biotech
Approval date: November 16, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DARZALEX is a drug used to treat patients with a form of blood cancer called multiple myeloma. It is for patients who have received at least three prior treatments for their cancer.
How is this drug used?
DARZALEX is given by a healthcare provider as an infusion into a vein. It is given according to a specific schedule.
What are the benefits of this drug?
In two trials, DARZALEX reduced the number of cancer cells or the amount of cancer in the body, also called tumor burden. In one trial, 29% of patients had a partial or complete reduction in their tumor burden, which lasted for an average of 7.4 months. In the second trial, 36% of patients had a partial or complete reduction in their tumor burden.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2. Efficacy Results for Trial 1
| N=106 | |
|---|---|
| Overall response rate (ORR) 95% CI (%) |
31 (29.2%) (20.8, 38.9) |
| Stringent complete response (sCR) | 3 (2.8%) |
| Complete response (CR) | 0 |
| Very good partial response (VGPR) | 10 (9.4%) |
| Partial response (PR) | 18 (17.0%) |
>ORR = sCR+CR+VGPR+PR
CI = confidence interval
In the second trial, Overall response rate was 36% (95% CI: 21.6, 52.0%) with 1 CR and 3 VGPR. The median time to response was 1 month (range: 0.5 to 3.2 months). The median duration of response was not estimable (range: 2.2 to 13.1+ months).
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: DARZALEX was similarly effective in men and women.
- Race: Most patients in the trials were white. Differences in response to DARZALEX among races could not be determined.
- Age: DARZALEX was similarly effective in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
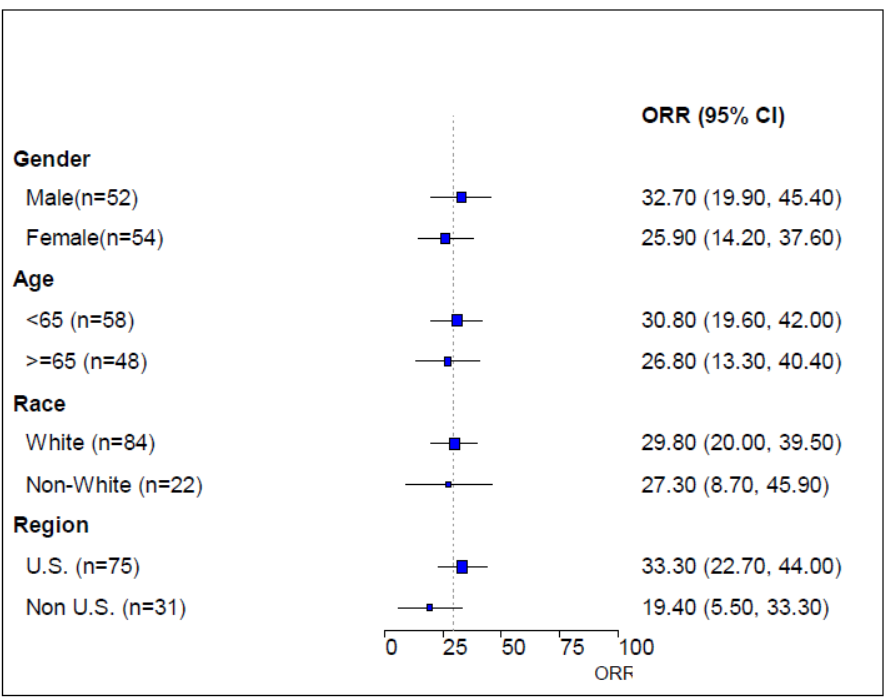
The figure below depicts the subgroup analyses for the larger trial done in 106 patients.
Figure 4. Trial 1 Subgroup Analyses on Objective Response Rates based on IRC Assessment; All Treated – 16 mg/kg Group
IRC=independent review committee
FDA Review
What are the possible side effects?
The most common side effects of DARZALEX were reactions related to the infusion of the drug, fatigue, nausea, back pain, fever and cough. DARZALEX may also result in low counts of infection-fighting white blood cells (lymphopenia, neutropenia, and leukopenia) or red blood cells (anemia) and low levels of blood platelets (thrombocytopenia).
What are the possible side effects?
The table below summarizes adverse events in the two clinical trials.
Table 3. Adverse reactions with incidence ≥10% in patients with multiple myeloma treated with DARZALEX 16 mg/kg
| System Organ Class | DARZALEX 16 mg/kg N=156 |
||
|---|---|---|---|
| Incidence (%) | |||
| Adverse Reaction | Any Grade | Grade 3 | Grade 4 |
| Infusion reactiona | 48 | 3 | 0 |
| General disorders and administration site conditions | |||
| Fatigue | 39 | 2 | 0 |
| Pyrexia | 21 | 1 | 0 |
| Chills | 10 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | |||
| Cough | 21 | 0 | 0 |
| Nasal congestion | 17 | 0 | 0 |
| Dyspnea | 15 | 1 | 0 |
| Musculoskeletal and connective tissue disorders | |||
| Back pain | 23 | 2 | 0 |
| Arthralgia | 17 | 0 | 0 |
| Pain in extremity | 15 | 1 | 0 |
| Musculoskeletal chest pain | 12 | 1 | 0 |
| Infections and infestations | |||
| Upper respiratory tract infection | 20 | 1 | 0 |
| Nasopharyngitis | 15 | 0 | 0 |
| Pneumoniab | 11 | 6 | 0 |
| Gastrointestinal disorders | |||
| Nausea | 27 | 0 | 0 |
| Diarrhea | 16 | 1 | 0 |
| Constipation | 15 | 0 | 0 |
| Vomiting | 14 | 0 | 0 |
| Metabolism and nutrition disorders | |||
| Decreased appetite | 15 | 1 | 0 |
| Nervous system disorders | |||
| Headache | 12 | 1 | 0 |
| Vascular disorders | |||
| Hypertension | 10 | 5 | 0/td> |
a Infusion reaction includes terms determined by investigators to be related to infusion, see below
b Pneumonia also includes the terms streptococcal pneumonia and lobar pneumonia
DARZALEX Prescribing Information
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of side effects was similar in men and women.
- Race: Most patients in the trials were white. Differences in side effects among races could not be determined.
- Age: The risk of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes subgroup analysis of treatment-emergent adverse events for the Safety Population in the clinical trials.
Table 4. Subgroup Analyses on Overview of Treatment-Emergent Adverse Events for the Pooled Population
| N | TEAE | Serious TEAE | |
|---|---|---|---|
| Sex Male Female |
84 72 |
82 (97.6%) 72 (100%) |
24 (28.6%) 26 (36.1%) |
| Race White Other |
119 37 |
117 (98.3%) 37 (100%) |
40 (33.6%) 10 (27%) |
| Age 18 to <65 years 65 to 74 years 75 and above |
86 54 16 |
86 (100%) 52 (96.3%) 16 (100%) |
26 (30.2%) 20 (37%) 4 (25%) |
TEAE-treatment-emergent adverse event
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved DARZALEX based primarily on evidence from 2 clinical trials in 156 patients with multiple myeloma who had received prior treatments. The trials were done in the United States, Canada, Europe, and Japan.
Figure 1 summarizes how many men and women were enrolled in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
Figure 2 and Table 1 summarize the percentage of patients by race enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 119 | 76% |
| Black or African American | 16 | 10% |
| Asian | 9 | 6% |
| Not Collected | 12 | 8% |
Clinical Trial Data
The Figure below summarizes the perentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographics for patients in the pooled clinical trials.
Table 5. Demographics for the Patients in the Two Pooled Clinical Trials
| Total N=156 |
|
|---|---|
| Demographic Subgroup | |
| Sex, n (%) | |
| Male | 84 (54) |
| Female | 72 (46) |
| Age Group, n (%) | |
| 18- <65 years | 86 (55) |
| ≥65 - <75 years | 54 (35) |
| >=75 years | 16 (10) |
| Race, n (%) | |
| White | 119 (76) |
| Black or African American | 16 (10) |
| Asian | 9 (5.8) |
| Other, unknown, and unreported | 12 (7.7) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 10 (6) |
| Not Hispanic or Latino | 135 (87) |
| Unknown | 11 (7) |
Clinical Trial Data
How were the trials designed?
Two trials were conducted in which all patients received DARZALEX. Treatment was continued until there were unacceptable side effects or the cancer progressed.
How were the trials designed?
Trial 1 was an open-label trial evaluating DARZALEX monotherapy in patients with relapsed or refractory multiple myeloma who had received at least 3 prior lines of therapy including a proteasome inhibitor and an immunomodulatory agent or who were double-refractory to a proteasome inhibitor and an immunomodulatory agent. In 106 patients, DARZALEX 16 mg/kg was administered with pre- and post-infusion medication. Treatment continued until unacceptable toxicity or disease progression.
Trial 2 was an open-label dose escalation trial evaluating DARZALEX monotherapy in patients with relapsed or refractory multiple myeloma who had received at least 2 different cytoreductive therapies. In 42 patients, DARZALEX 16 mg/kg was administered with pre- and post-infusion medication. Treatment continued until unacceptable toxicity or disease progression.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.