Formulating Drug Products for Optimized Absorption: Elucidating Amorphous Solid Dispersions
CDER scientists are seeking ways to improve the bioavailability of drugs that on their own do not dissolve well in water. Recent CDER research explores the potential for using amorphous solid dispersions to formulate generic drug products that may include ingredients that are poorly water-soluble.
Introduction
Amorphous solid dispersions (ASDs) are a popular mechanism for enhancing the solubility and bioavailability of drugs that are poorly soluble in water. Important drugs that treat cancer, cystic fibrosis, and organ transplant rejection, to name a few, use ASDs in their formulation to help overcome the solubility limitations of drugs when they are administered orally. Recent research conducted by FDA’s Office of Generic Drugs in the Center for Drug Evaluation and Research explored the mechanistic understanding and prediction of in vivo performance of ASD drug products. Findings from the research create a valuable foundation for understanding the impact of biologically relevant media on solution phase behavior of poorly soluble drugs which can assist in the development of generic drug products that will use ASDs. The research results are also helpful for the regulatory assessment and evaluation of the bioinequivalence risk of the test drug products compared to the reference drug products.
Background
Orally administered drugs must dissolve in the gastrointestinal tract before they can be absorbed into the body’s circulation, and yet 40% of approved drugs are considered insoluble; nearly 90% of developmental compounds are considered poorly soluble.1 As a result, formulation scientists often formulate drugs to overcome solubility limitations. Many approaches, such as co-formulating with solubilizing agents, using salts, co-crystals, and amorphous solid dispersions, have been employed to enhance solubility.2 In the past decade, there has been an increasing realization that certain active pharmaceutical ingredients may, by virtue of their inherent physical and chemical properties, become “saturated” in solution within the gastrointestinal tract at a concentration insufficient to achieve the desired absorption into the body. In such instances, it is necessary to increase the number of molecules in the solution above the saturation point.3 Researchers have therefore explored ways to generate “supersaturated solutions” and thereby improve the rates of drug delivery from the GI tract into the blood stream,4particularly when the drug of interest has been shown to be otherwise poorly soluble.3
The process of crystallization from a supersaturated solution can be explored through physiologically based pharmacokinetic (PBPK) modeling,5,6 and the research shows that a significant proportion of poorly water-soluble drugs go through an intermediate state prior to crystallization, first forming an amorphous phase, which is often colloidal, via the process of liquid-liquid phase separation (LLPS).7 LLPS is a thermodynamically driven, reversible phenomenon consisting in de-mixing into two distinct liquid phases, with different solute concentrations. The phases subsequently undergo crystallization over a physiologically relevant time frame. Characterizing the types of phase transitions is of critical importance because the precipitation outcome (i.e., formation of solid material from substance in solution) is directly linked to the supersaturation profile; if the precipitate is amorphous, the solution remains supersaturated.8 This in turn is expected to influence the predicted absorption kinetics. Furthermore, the precipitation outcome can be influenced by the presence of other components, either present in the formulation,9,10 or endogenous substances (e.g., bile salts).11
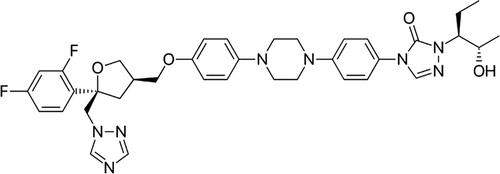
CDER scientists evaluated the landscape of precipitation outcomes and kinetics for a model drug that is poorly water-soluble. Posaconazole (PCZ) (Figure 1), a triazole antifungal drug used to treat invasive fungal infections, was selected as the model compound. PCZ is a poorly water-soluble and weakly basic.12−14 With different gastric concentrations, induced by different administration media, PCZ has been shown to lead to different levels of supersaturation, precipitation, and exposure.15 Given these interesting in vivo observations, the weakly basic nature of the drug, its prescribed dose (200–300 mg 2–3 times per day), and the different commercial formulations available (suspension vs ASD tablet with an enteric polymer), this compound offered several study areas in terms of understanding in vitro phase behavior.
Figure 1. Chemical structure of PCZ.
In vitro experiments were designed, first defining the phase diagrams for this system, and then evaluating the phase behavior and nucleation kinetics. Different extents of supersaturation were evaluated using physiologically relevant media.
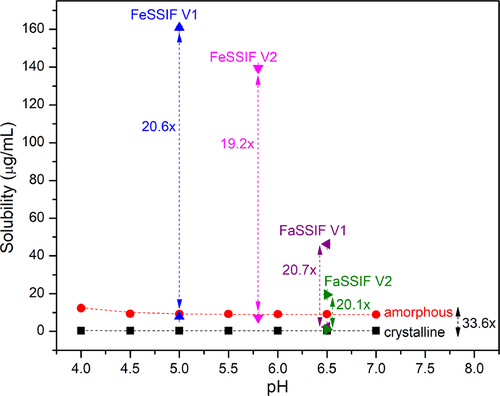
Figure 2. Modified phase diagram with phase boundaries for fasted and fed state intestinal fluid. For each biorelevant medium, the lower and higher data points represent the crystalline and amorphous solubility, respectively.
Mapping Possible Phase Transitions
Since the driving force for passive diffusion through the intestinal membrane is proportional to the concentration of the uncharged molecule, the supersaturation ratio indicates the potential improvement in the absorption rate. Furthermore, as amorphous solubility is the limit of the maximum achievable supersaturation, above which LLPS is thermodynamically favorable, knowledge of the supersaturation ratio also allows prediction of the possible phase transitions.
Conclusion
Understanding the supersaturation and precipitation behavior of poorly water-soluble compounds in vivo, and the impact on oral absorption, is critical to designing consistently performing drug products with desired absorption into the body. Weak basic compounds are of particular importance in this context because they have an inherent tendency to undergo supersaturation in vivo upon exit from the stomach and entry into the small intestine because of their pH-dependent solubility. Through the extensive characterization of the solution phase behavior of PCZ, FDA has demonstrated that the amorphous and crystalline solubilities follow the same trend with pH, where the maximum achievable supersaturation ratio across the pH range tested was approximately 34. Defining the phase diagram in different media enables mapping of potential phase transitions, including LLPS and crystallization during transit through various regions of the GI tract which vary with respect to pH and endogenous surfactant concentration and composition. Furthermore, it was demonstrated that biological surfactants, present in simulated biorelevant media, had a notable impact not only on the solubility but also on the drug nucleation kinetics. Our findings are valuable for understanding the impact of biologically relevant media on solution phase behavior of poorly soluble drugs, which may help improve prediction of the in vivo behavior of supersaturating drug products.
How does this research improve generic drug development and support their approval?
This research explores the current mechanistic understanding and prediction of in vivo performance of amorphous solid dispersions drug products for test and reference drug. This research may be valuable for the development of generic drug products used to treat diseases such as cancer, cystic fibrosis, and heart, liver, and lung transplant rejection. This better understanding will also be critical to guide the regulatory assessment and evaluation for test drug products regarding the risk of not being bioequivalent compared to their reference standard.
This Impact Story is based on: Van Duong T, Ni Z, Taylor LS. Phase behavior and crystallization kinetics of a poorly water-soluble weakly basic drug as a function of supersaturation and media composition. Mol Pharm. 2022 Apr 4;19(4):1146-1159. doi: 10.1021/acs.molpharmaceut.1c00927. Epub 2022 Mar 23. PMID: 35319221.
References
- Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010 Nov;62(11):1607-21. doi: 10.1111/j.2042-7158.2010.01030.x. PMID: 21039545.
- Williams HD, Trevaskis NL, Charman SA, Shanker RM, Charman WN, Pouton CW, Porter CJ. Strategies to address low drug solubility in discovery and development. Pharmacol Rev. 2013 Jan;65(1):315-499. doi: 10.1124/pr.112.005660. PMID: 23383426.
- Brouwers J, Brewster ME, Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci. 2009 Aug;98(8):2549-72. doi: 10.1002/jps.21650. PMID: 19373886.
- Miller JM, Beig A, Carr RA, Spence JK, Dahan A. A win-win solution in oral delivery of lipophilic drugs: supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol Pharm. 2012 Jul 2;9(7):2009-16. doi: 10.1021/mp300104s. Epub 2012 Jun 20. PMID: 22632106.
- Ilevbare GA, Taylor LS. Liquid–liquid phase separation in highly supersaturated aqueous solutions of poorly water-soluble drugs: implications for solubility enhancing formulations. Cryst Growth Des. 2013, 13, 1497– 1509, DOI: 10.1021/cg301679h.
- Taylor LS, Zhang GGZ. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv Drug Deliv Rev. 2016 Jun 1;101:122-142. doi: 10.1016/j.addr.2016.03.006. Epub 2016 Mar 22. PMID: 27013254.
- Longhi S, Brocca S, Grandori R, Uversky, V. Liquid–Liquid Phase Separation. Encyclopedia. Available online: https://encyclopedia.pub/entry/7187 (accessed on 16 June 2022).
- Raina SA, Zhang GGZ, Alonzo DE, Wu J, Zhu D, Catron ND, Gao Y, Taylor LS. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J Pharm Sci. 2014 Sep;103(9):2736-2748. doi: 10.1002/jps.23826. Epub 2013 Dec 30. PMID: 24382592.
- Ilevbare GA, Liu H, Edgar KJ, Taylor LS. Maintaining supersaturation in aqueous drug solutions: impact of different polymers on induction times. Cryst Growth Des. 2012, 13, 740– 751, DOI: 10.1021/cg301447d.
- Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001 Jan 16;212(2):213-21. doi: 10.1016/s0378-5173(00)00610-4. PMID: 11165079.
- Chen J, Mosquera-Giraldo LI, Ormes JD, Higgins JD, Taylor LS. Bile salts as crystallization inhibitors of supersaturated solutions of poorly water-soluble compounds. Cryst Growth Des. 2015, 15, 2593– 2597, DOI: 10.1021/acs.cgd.5b00392.
- Courtney R, Radwanski E, Lim J, Laughlin M. Pharmacokinetics of posaconazole coadministered with antacid in fasting or nonfasting healthy men. Antimicrob Agents Chemother. 2004 Mar;48(3):804-8. doi: 10.1128/AAC.48.3.804-808.2004. PMID: 14982768; PMCID: PMC353067.
- Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2004 Feb;57(2):218-22. doi: 10.1046/j.1365-2125.2003.01977.x. PMID: 14748822; PMCID: PMC1884431.
- Wieser J, Pichler A, Hotter A, Griesser U, Langes, C. A crystalline form of posaconazole. EP 2141159 A1, 2010.
- Hens B, Brouwers J, Corsetti M, Augustijns P. Supersaturation and Precipitation of Posaconazole Upon Entry in the Upper Small Intestine in Humans. J Pharm Sci. 2016 Sep;105(9):2677-2684. doi: 10.1002/jps.24690. Epub 2016 Feb 22. PMID: 26505884.