Human Factors and Medical Devices
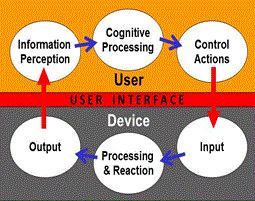
Human factors/usability engineering focuses on the interactions between people and devices. Figure 1 presents a model of the interactions between a user and a device, the processes performed by each, and the user interface between them. The critical element in these interactions is the device user interface, depicted as the red zone.
Figure 1: Device User Interface in Operational Context (adapted from Redmill and Rajan, 1997).
To understand the user-device system, it's important to understand the ways that people:
- Perceive information from the device,
- Interpret the information and make decisions about what to do, and
- Manipulate the device, its components, and/or its controls. (e.g., modify a setting, replace a component, or stop the device).
It's also important to understand the ways that devices:
- Receives input from the user, and then
- Responds and provides feedback to the user about the effects of their actions.
Human factors/usability engineering is used to design the user-device interface. The user interface includes all components with which users interact while preparing the device for use (e.g., unpacking, set up, calibration), using the device, or performing maintenance (e.g., cleaning, replacing a battery, making repairs).
Why is Human Factor Engineering important to medical devices?
For medical devices, the most important goal of the human factors/usability engineering process is to minimize use-related hazards and risks and then confirm that these efforts were successful and users can use the device safely and effectively.
Specific beneficial outcomes of applying human factors/usability engineering to medical devices include:
- Safer connections between device components and accessories (e.g., power cords, leads, tubing, cartridges),
- Improved controls and displays interaction,
- Better user understanding of the device's status and operation,
- Better user understanding of a patient's current medical condition,
- More effective alarm signals management,
- Easier device maintenance and repair,
- Reduced user reliance on user manuals,
- Reduced need for user training and retraining,
- Reduced risk of use error,
- Reduced risk of adverse events, and
- Reduced risk of product recalls.
Contact FDA
CDRH Human Factors Team
Office of Product Evaluation and Quality
Center for Devices and Radiological Health
Food and Drug Administration
10903 New Hampshire Avenue
WO66, Room 2502
Silver Spring, MD 20993