Quality Systems
Guide to Inspections of Quality Systems
August 1999
Credits Page
Adobe PDF version for printing
Foreword
This document provides guidance to the FDA field staff on a new inspectional process that may be used to assess a medical device manufacturer's compliance with the Quality System Regulation and related regulations. The new inspectional process is known as the "Quality System Inspection Technique" or "QSIT". Field investigators may conduct an efficient and effective comprehensive inspection using this guidance material which will help them focus on key elements of a firm's quality system.
Note: This manual is reference material for investigators and other FDA personnel. The document does not bind FDA and does not confer any rights, privileges, benefits or immunities for or on any person(s).
Table of Contents
- Performing Subsystem Inspections
- Pre-announced Inspections
- Getting Started
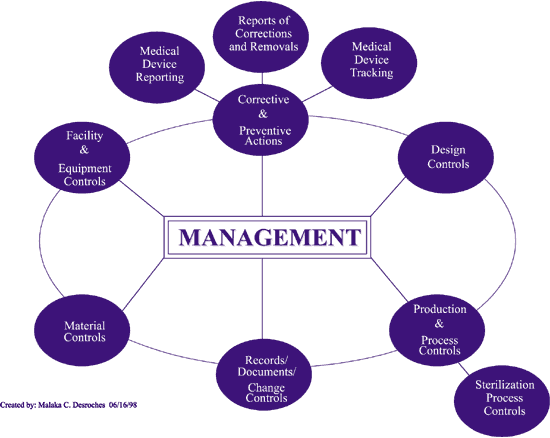
- Management Controls
- Design Controls
- Corrective and Preventive Actions (CAPA)
- Production and Process Controls (P&PC)
- Sampling Plans
This reference is intended to be used in conjunction with the:
- Compliance Program Guidance Manual for Inspection of Medical Device Manufacturers (CP 7382.845).
- Investigations Operations Manual (IOM).
- Code of Federal Regulations, Title 21 (21 CFR) Part 820 Quality System Regulation; Part 803 Medical Device Reporting; Part 806 Medical Device Corrections and Removals; Part 821 Medical Device Tracking.
- Compliance Policy Guides (CPG) for devices (Sub Chapter 300).
- Guideline on General Principles of Process Validation, FDA, May 1987.
Other references include:
- The Federal Food, Drug, and Cosmetic Act; The Safe Medical Devices Act (SMDA) of 1990 and the Medical Device Amendments of 1992.
- Medical Device Quality Systems Manual: A Small Entity Compliance Guide.
- The FDA Worldwide Quality System Requirements Guidebook for Medical Devices.
- Other device specific guidance documents prepared by CDRH for the medical device industry.
- FDA Recognized Standards.
These additional guidances are posted to the CDRH Internet World Wide Web Home Page at http://www.fda.gov/medicaldevices/default.htm. See IOM Chapter 10, References, for additional information.